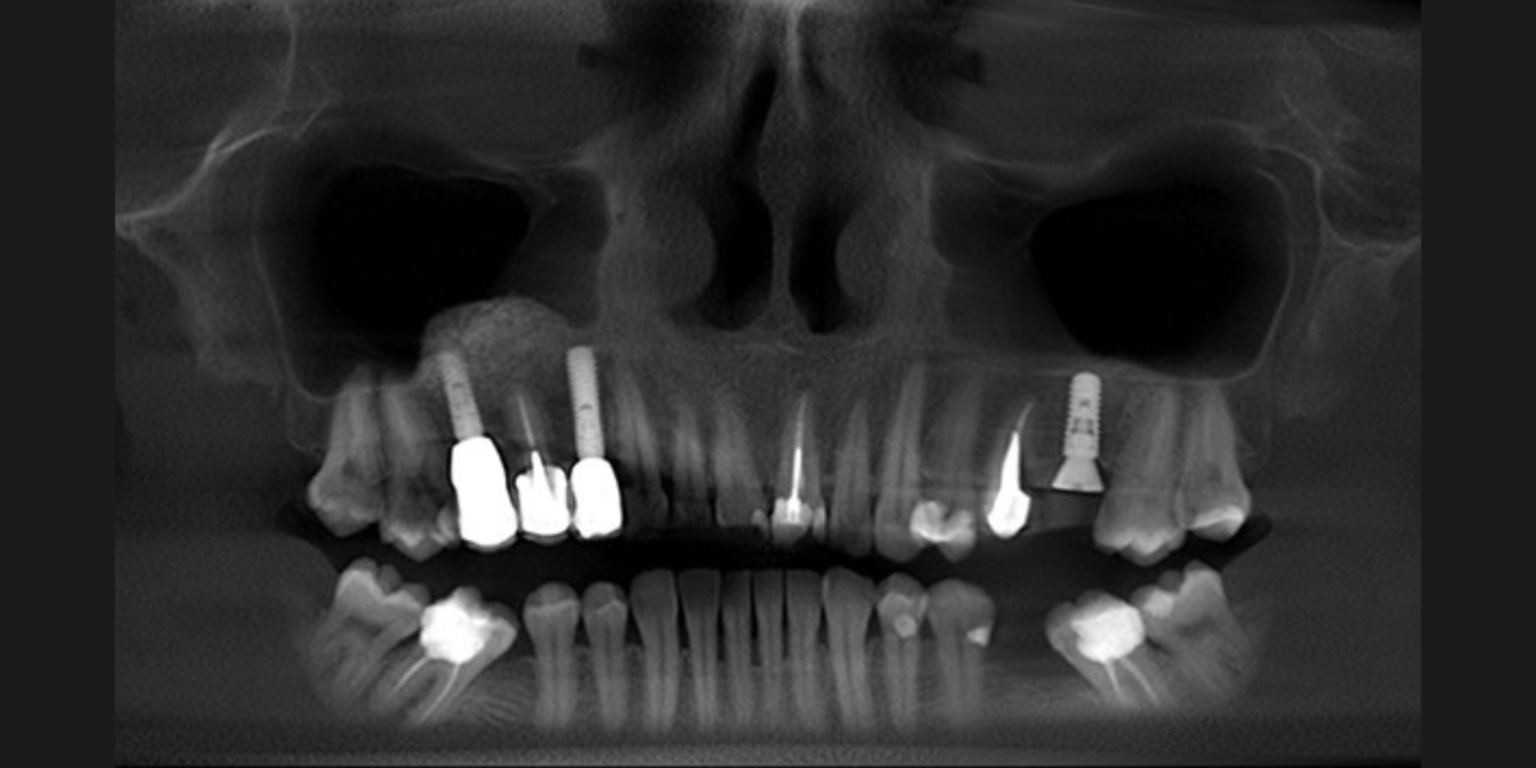
Regenerative dentistry
As life expectancy increases in modern years, so does the importance of maintaining a healthy natural dentition. Towards this end, regenerative therapies play a central role, however without always achieving a predictable treatment outcome. The diversity of clinical cases, the biology of the patient, and the diversity of the biomaterials as well as the bioactive agents available in the market, often fail to demonstrate clinically significant differences in outcomes. Therefore, clinicians are asked to keep up to date with advances made in evidence-based regenerative methodologies for each clinical indication in quest of a safe, effective, economic, and timely treatment. Cell therapy and tissue engineering comprise a promising tool in regenerative dentistry and are still under investigation for their potential to safely and effectively restore damaged tissues in the oral cavity.
Cell therapy and tissue engineering
Treatment technologies employing biomaterials, bioactive agents (Sanz et al. 2019), and sophisticated flap designs have been introduced in periodontal therapy based on their potential to activate the intrinsic healing potential of periodontal tissues and modulate the cellular events required for periodontal regeneration (Susin et al. 2015). Tissue engineering based on the triad of mesenchymal stem cells (MSCs), biomolecules and biomaterial scaffolds has been considered as an alternative for periodontal (Hynes et al. 2012) and bone regeneration (Shanbhag et al. 2019). Mesenchymal stem cells have demonstrated multipotency, immune modulatory and trophic properties by secreting bioactive factors and homing on sites of injury or disease to regenerate tissues. These cells can be isolated from a small tissue harvest and can be expanded to millions for clinical use in culture conditions compatible with “Good Manufacturing Practices” (c-GMP) (Bakopoulou et al. 2017; Rojewski et al. 2019). The origin of MSCs seems to be central for a successful clinical application and several sources including bone marrow aspiration or tissue harvest from the oral cavity (e.g., alveolar bone, gingivae, dental pulp) have been investigated for their regenerative potential (Egusa et al. 2012; Brennan et al. 2017). Biomaterial scaffolds also play a significant role in tissue regeneration (Seebach et al. 2010), as they provide a favorable micro-environment to guide tissue regeneration and should be carefully chosen for a particular clinical application. Bioactive factors enhance the regenerative capacity of damaged tissues by improving cellular chemo-attraction, differentiation and proliferation, in essence regulating important cellular events involved in wound healing (Trombelli and Farina 2008).
Clinical applications of MSCs
Periodontal and implant cell therapy approaches
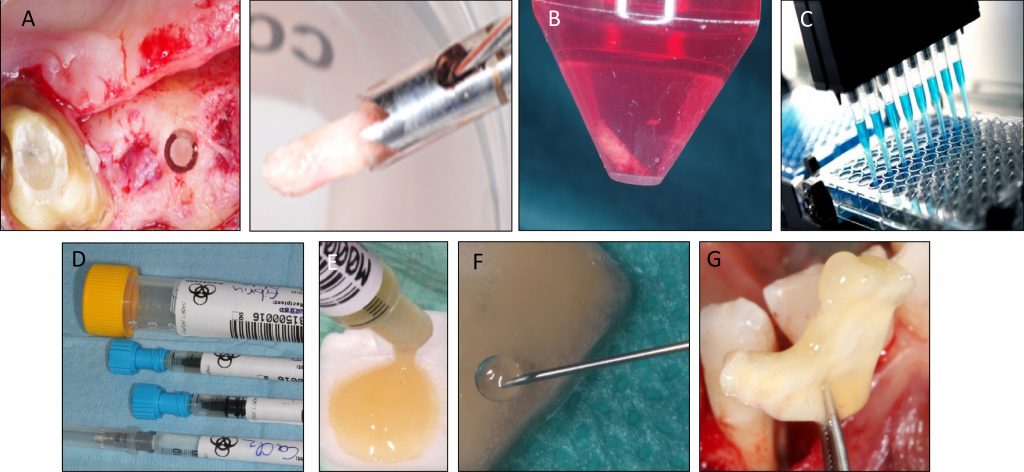
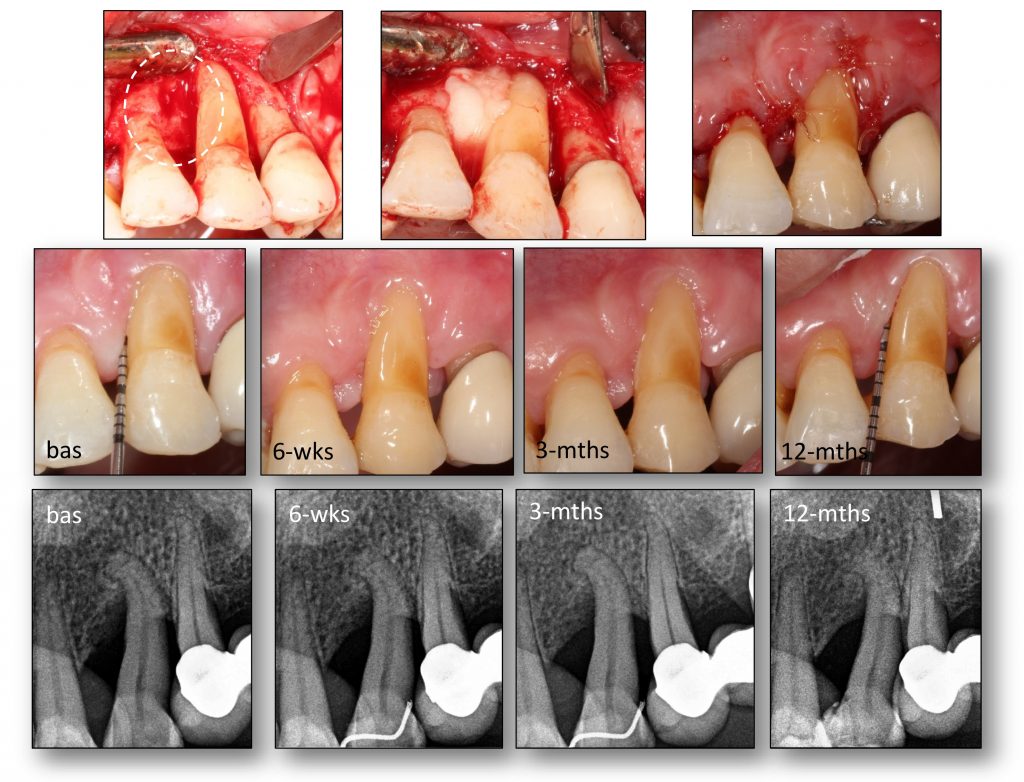
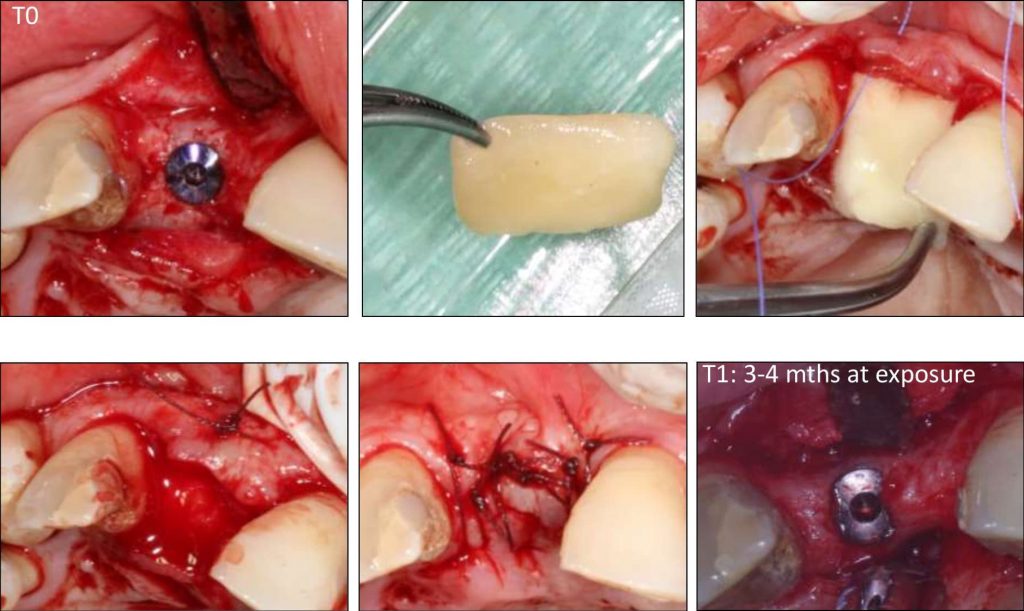
Tissue engineering by means of autologous mesenchymal stem cells, platelet concentrates and collagen scaffolds – the so-called “biocomplex” – has been designed to reconstruct periodontal intrabony defects (Apatzidou et al. 2021). This tissue-engineered product has been also used in implant therapy to stabilize the soft and hard tissues around an implant aiming to lead to predictable esthetic outcomes and stable marginal bone levels during its lifetime (Apatzidou et al.; IADR general session 2019; abstract ID: 3175694). An operator-friendly clinical approach has been developed to assemble chair-side the different constituents of the biocomplex (autologous alveolar bone marrow MSCs, platelet concentrates, collagen scaffold) following simple procedures and within a short time to avoid delays in the operating room. In more detail, based on this protocol an osseous biopsy of ~3 ml in volume was collected from the alveolar bone under local anaesthesia during routine dental procedures (tooth extraction, collection from the maxilla tuberosity, implant placement). After thorough rinsing with saline, it was transported on cool packs to a c-GMP-authorized facility, where within a period of 3 weeks 10 million cells were produced for clinical use after passing stringent quality controls. The cells were suspended in autologous fibrin/platelet lysate (aFPL) and delivered to the clinic in a plastic syringe with a cap replacing the needle. In the clinical setting, the suspension of cells in aFPL was gently loaded into a collagen fleece (Parasorb® fleece, Resorba, Germany) allowing 5 minutes to rest and later the construct was crosslinked by CaCl2 (Fig. 1). Before the biocomplex solidified (<10 min), it was carefully applied at the surgical site precisely fitting with the surrounding tissues. In the periodontal application, the biocomplex was carefully placed into the osseous defect avoiding overfill and was covered by the gingival flaps utilizing a Minimal Access Flap (Apatzidou et al. 2021) (Fig. 2); in the implant application the implant was surgically placed in pristine bone and the biocomplex was stabilized onto the reflected full-thickness flaps either on the lingual or the buccal aspect using a horizontal mattress suture. Care was taken to cover the shoulder of the implant and also extend on either side of the alveolar ridge by 3-5 mm and subsequently, the implant with the overlying biocomplex were submerged (Apatzidou et al; IADR general session 2019; abstract ID: 3175694) (Fig. 3).
The biocomplex as described here comprises a well-defined, reproducible and safe tool in regenerative dentistry that was applied in periodontal therapy to reconstruct intrabony periodontal defects, and in implant therapy to optimize tissue healing following implant placement in pristine bone. In order to better understand the impact that the engrafted cells have on periodontal and osseous wound healing, the biocomplex was not combined with any other graft materials that may mask the real healing events that take place. This emerging evidence will assist in the design of future studies and hopefully optimize cell therapy applications in several clinical applications.
Alveolar bone tissue engineering
Atrophy of the alveolar process is a common finding in partially edentulous patients who often have to go through complicated surgeries and bone augmentation procedures to receive implant-borne prostheses. Although autologous bone grafts are considered the gold standard in alveolar bone regeneration there are several drawbacks to their use. Prolonged time in the operating theater with the associated costs, limited quantities of harvest, morbidity at the donor site and rapid resorption following transplantation comprise the main limitations of autologous bone grafting.
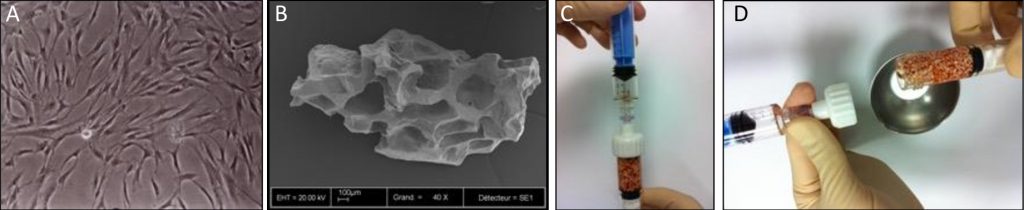
A: Culture-expanded MSCs
B: Biphasic calcium phosphate granules
C & D: Associated in the operating room
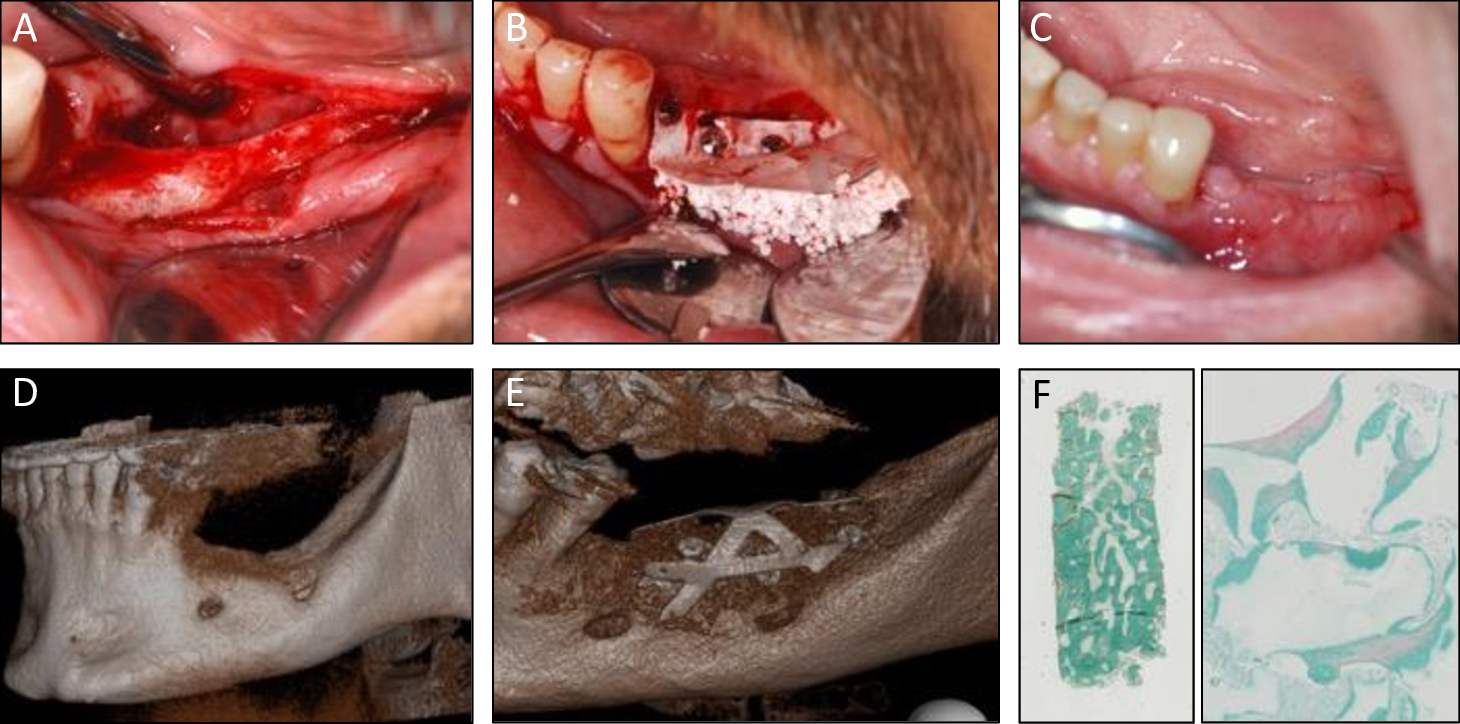
Combinations of autologous bone marrow MSCs and biomaterials have been investigated for their safety and efficacy in bone regeneration in mice suggesting this approach as an alternative to conventional bone augmentation techniques (Brennan et al. 2014). More recently, a clinical study funded by the European Commission has been successfully conducted in 11 patients by using culture-expanded MSCs and biomaterials for alveolar bone augmentation through the REBORNE project (Gjerde et al. 2018). As shown in Fig. 4, autologous cells were isolated from bone marrow aspirates and amplified in culture before quality controls and shipment to the clinics, where they were associated with the biomaterial granules prior to implantation in patients (Rojewski et al. 2019). The safety and efficacy of this novel approach were documented by clinical photographs, computed tomography and histology of osseous biopsies collected from the experimental site upon implant placement (Fig. 5). Although cell therapy is considered to be promising in certain clinical indications, its superiority in alveolar bone regeneration over autologous bone grafts however still remains under investigation (Shanbhag et al. 2019). Towards this end, a randomized multi-center controlled clinical trial funded by the European Commission is currently in progress and aims to compare the efficacy of cell therapy in alveolar bone augmentation over autologous bone grafting based on a sample size of 150 partially edentulous patients [www.maxibone.eu].
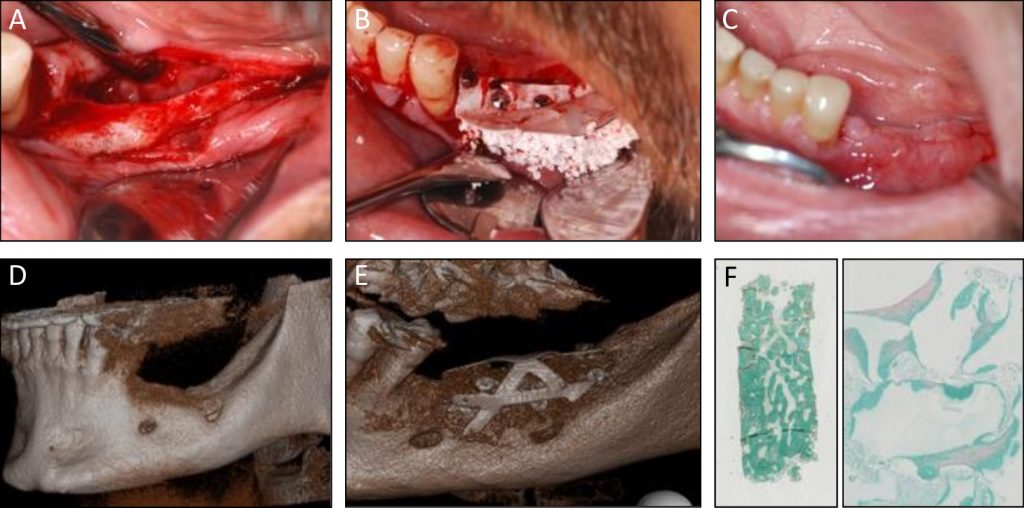
A, B & C: Clinical images at flap opening, at grafting MSC and biomaterials, and at 5 months post-surgery
D: Pre-operative
E: 5 months post-operative scanner
F: Histology of the core biopsy showing bone regeneration between granules (Goldner trichrome staining)
Ethics and regulatory policies in cell therapy applications
Major limitations of cell therapy are related to ethical and regulatory considerations as MSCs are considered an advanced therapy medicinal product (ATMP) by the European Medicine Agency (EMA). As cell therapy is regarded as a drug, it is mandatory to complete pre-clinical studies and phase II and phase III clinical trials prior to market authorization by the EMA. Furthermore, the ATMP must be handled and produced under strict GMP regulations in an approved pharmaceutical facility in clean rooms by qualified personnel. Prior to authorizing a clinical trial, the review of a dossier including the clinical protocol and patient recruitment, data storage, the manufacturing procedures and release criteria is required by national regulatory agencies. Consequently, the costs of the production, quality controls and transportation of cell therapy products are high, estimated at €20,000/patient, thus being approximately tenfold higher than conventional autografts. However, the involvement of blood transfusion institutes across Europe instead of private laboratories may decrease the costs of cell therapy. Nevertheless, handling living cell therapy products also poses a logistical problem for general dental practices as it has to be synchronized with the laboratory facility with regard to the timing of cell delivery and transplantation. Allogeneic cells derived from other donors, cryopreserved in certified tissue banks and ready to be shipped upon demand may be an alternative in regenerative dentistry. It is expected that this allogeneic cell therapy will decrease the price for each preparation by approximately €5,000 resulting in a more competitive and cost-effective treatment than the autologous pathway, although the clinical demonstration of safety and efficacy remains to be assessed.
Translation to general practice
Cell therapy and tissue engineering have been shown to have advantages in orofacial regeneration over conventional treatment procedures in cases where the defects are of complex anatomical configuration. There are already numerous human applications in sinus augmentation, reconstruction of large and smaller alveolar bone defects and periodontal defects. However, the relevant studies are of small sample size and short follow-up period and are mainly case reports/series or case control, while randomized controlled clinical trials are scarce. Cell therapy has been also tested in implant surgery to augment the alveolar bone for optimal implant placement to safeguard marginal bone levels and soft-tissue integrity following surgical placement in pristine bone (Apatzidou et al; IADR general session 2019; abstract ID: 3175694) with promising treatment outcomes. However, there is a void in the literature regarding the use of this novel treatment approach in the challenging cases of peri-implantitis.
Given the logistical, regulatory and economic constraints of cell therapy that impede its routine use in the clinical setting, additional years of research are required before cell therapy comes into a clinical reality. Treatment technologies based on cell components, such as the secretome and/or extracellular vesicles of the MSCs or less expensive approaches of allogeneic cells combined with sophisticated scaffolds and/or biomaterials might be future therapeutic alternatives. Interestingly, a less complex procedure having “minimal manipulation” in the form of a micro-graft (Trovato et al. 2015), has been also introduced in periodontal therapy to avoid culture-expanding procedures and pertinent regulatory and practical limitations (Moreno Sancho et al. 2019).
Given the fact that regenerative dentistry is still in its infancy and still evolving, fundamental research and dental industries should work closely together to overcome all these hurdles and speed up the development of cell therapy products for the benefit of a large number of patients. However, the cost-effectiveness of cell therapy is of paramount importance and before this approach passes into the phase of large-scale production, cost-effectiveness should be carefully determined. In future, one can envision a chair side bioreactor that would process and produce a patient-specific anatomical synthetic bone graft comprising allogeneic cells loaded into biomaterials and tailored for regenerative dentistry applications.
Related content by the ITI: