Background
The introduction of dental implants into contemporary dental practice presents one of the major breakthroughs for the replacement of missing teeth. Dental implants have certainly advanced oral rehabilitation and patient-related quality of life in partially and fully edentulous patients, alongside high survival rates and success rates (Pjetursson, 2014). Yet, complications around dental implants and implant-borne restorations occur. They are mainly the consequence of one of the following factors:
- Clinician-related factors (poor treatment planning and performance, inadequate 3D implant positioning)
- Patient-specific factors (local and/or systemic conditions, mainly periodontal disease, uncontrolled systemic diseases, smoking)
- Biological factors (inflammation)
- Biomechanical factors
Long-term clinical studies have demonstrated that the complication and failure rates of implants increase over time (Ting, 2018).
The most common complication around dental implants and the primary reason for implant loss is a biological complication known as peri-implantitis. Implant-related biological complications are a consequence of biofilm-induced inflammation in the surrounding soft and hard tissues, presented as two distinct entities: peri-implantitis and peri-implant mucositis.
Whereas peri-implant mucositis is a reversible inflammatory lesion of the peri‐implant mucosa with the absence of progressive marginal bone loss (Heitz-Mayfield, 2018), peri-implantitis is characterized by inflammation of the adjacent circumferential connective tissues and escalating loss of the supporting bone, that, if not recognized and treated, might also lead to implant loss (Schwarz, 2018).
It has been shown that implants tend to show higher complication rates in periodontally compromised patients, thus the history of periodontitis exhibits a risk factor for the onset and progression of peri-implant diseases (Safi, 2010, Schou, 2006). Although peri-implantitis bears many similarities to periodontitis, it is well known that peri-implantitis has a faster, non-linear, and more aggressive progression (Schwarz, 2018). However, the whole process behind the etiopathogenesis of this disease, as well as its treatment, have not been fully clarified yet.
Etiological factors
Biofilms are complex microbial communities that grow on various surfaces in nature. The oral microbiota is prone to polymicrobial biofilm formation, particularly on the hard mineralized tooth surfaces, which may have an impact on oral health and consequently on disease progression. Specific biofilms with predominant anaerobic species can cause inflammation of the adjacent tooth-supporting tissues, leading to destructive periodontal disease and ultimately to tooth loss. In this regard, implants and their components might also become exposed to biofilm formation.
Peri-implantitis-associated biofilms colonizing exposed implant surfaces are composed of a wide variety of microbial species that cause inflammation and progressive tissue destruction (Belibasakis, 2015). The oral cavity is a complex ecosystem that harbors a vast number of different bacterial species, as well as other types of microbes such as viruses and fungi (Wade, 2013). These microbiota usually live in symbiosis with the host (Dewhirst, 2010), although in certain conditions they can trigger a number of diseases. Qualitative and/or quantitative shifts of the oral microbiome can lead to dysbiosis, known to be responsible for the development of microbially-related pathologies.
As with periodontal pockets, peri-implant pockets provide an ideal habitat for the proliferation of microbes supported by anerobic conditions and the presence of microbial nutrients in the gingival/peri-implant crevicular fluid (Lasserre, 2018). Furthermore, the literature has also reported on the considerable prevalence of viruses in patients with peri-implantitis (Brown, 2020). Traditionally, peri-implantitis was regarded as microbially equal to periodontitis, and translocation of periodontal pathogens into the peri-implant crevice was considered a critical factor in disease causation. However, although there are many similarities, recent evidence suggests that the peri-implant and overall periodontal ecosystems differ in many ways (Robitaille, 2018).
Biofilms associated with peri-implant infection (peri-implant mucositis and peri-implantitis) have been studied extensively using various microbiological techniques. Most studies found the composition of the submucosal microbiota to be similar to that found in chronic periodontitis. Some studies, however, have identified high numbers of microorganisms not commonly associated with periodontal disease. The microbiota associated with peri-implant mucositis appear to be similar to those associated with peri-implantitis (Máximo, 2009), suggesting that supramucosal plaque formation and the development of peri-implant mucositis could potentially be the precursors to peri-implantitis.
Preventive measures
Clinical guidelines have been adapted from ITI Treatment Guide Vol. 8. entitled Biological and Hardware Complications in Implant Dentistry.
Preventive measures before implant placement
- Residual periodontal pockets are a risk for peri-implant disease and implant loss. Completion of active periodontal therapy with the aim to eliminate the residual pockets is a mandatory step in periodontal patients prior to implant treatment
- In cases of persisting periodontal inflammation (PD ≥ 5 mm, BOP+, PI > 20%,) – re-treatment and periodontal re-evaluation are recommended before implant placement.
- In patients with the clinical entity known as aggressive periodontitis, a more frequent maintenance program is essential, known as supportive periodontal treatment (SPT)
- During implant treatment planning, factors to be considered that may result in biological complications include insufficient keratinized mucosa and bone volume at the implant recipient site, implant and tooth proximity, three-dimensional implant position, prosthesis design and cleansability.
Preventive measures after implant placement (Heitz-Mayfield, 2014)
- All oral healthcare providers, including undergraduate students, should be trained to recognize clinical signs of peri-implant pathology and maintain or re-establish peri-implant health.
- After delivery of the definitive implant-supported prosthesis, it is advised to probe around dental implants to record clinical measurements and to additionally document dental implants by means of clinical photographs and radiographs
- During SPT, updating medical and dental history and carrying out a clinical inspection of the implant-supported prosthesis including the evaluation of iatrogenic factors
- Regular diagnostic monitoring of the peri-implant tissues should include assessment of plaque presence (PI), probing depth (PD), extent of bleeding on gentle probing (BoP), and/or suppuration (SUP).
- In the presence of clinical signs of disease, an appropriate radiograph is indicated to detect radiographic bone-level changes compared to previous examinations. PD changes should be followed in relation to a marked fixed point.
- A recall frequency of at least once per year is recommended unless systemic and/or local conditions require more frequent intervals.
- In cases of peri-implant mucositis – OH reinforcement + mechanical debridement
- In cases of peri-implantitis – early treatment needed to prevent further progression
Diagnosis
Thanks to a joint workshop held by the European Federation of Periodontology (EFP) and American Academy of Periodontology (AAP) in Chicago, USA in 2017, a novel uniform definition of peri-implant diseases and conditions is now available .
How can we diagnose peri-implant conditions and diseases?
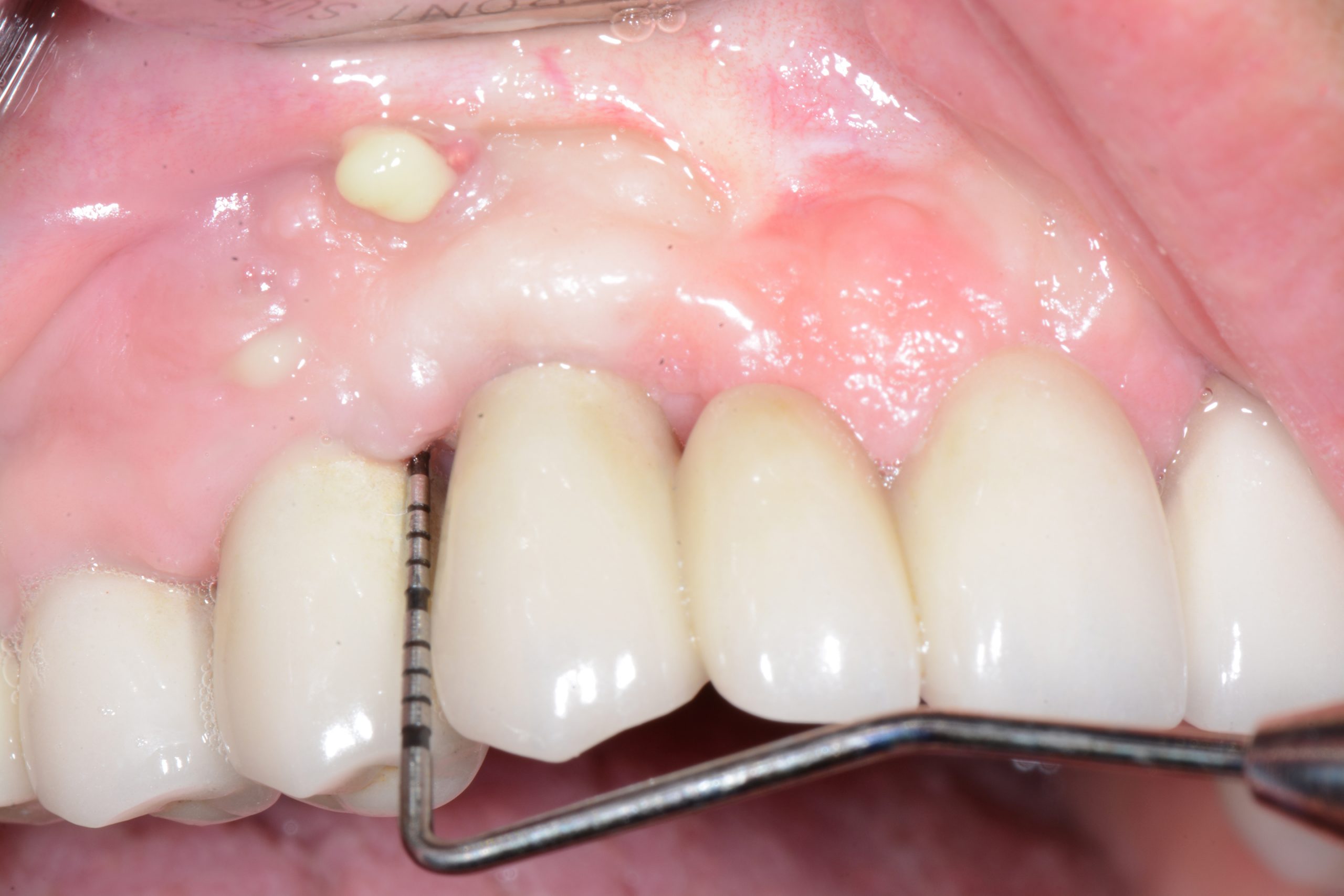
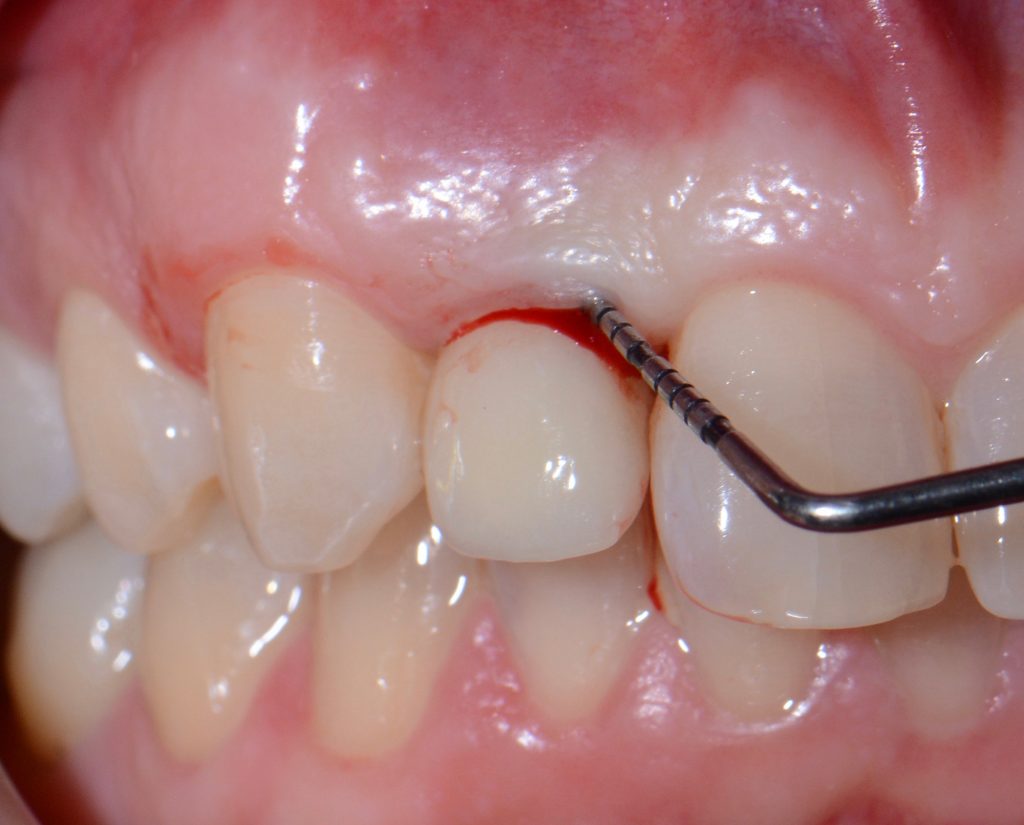
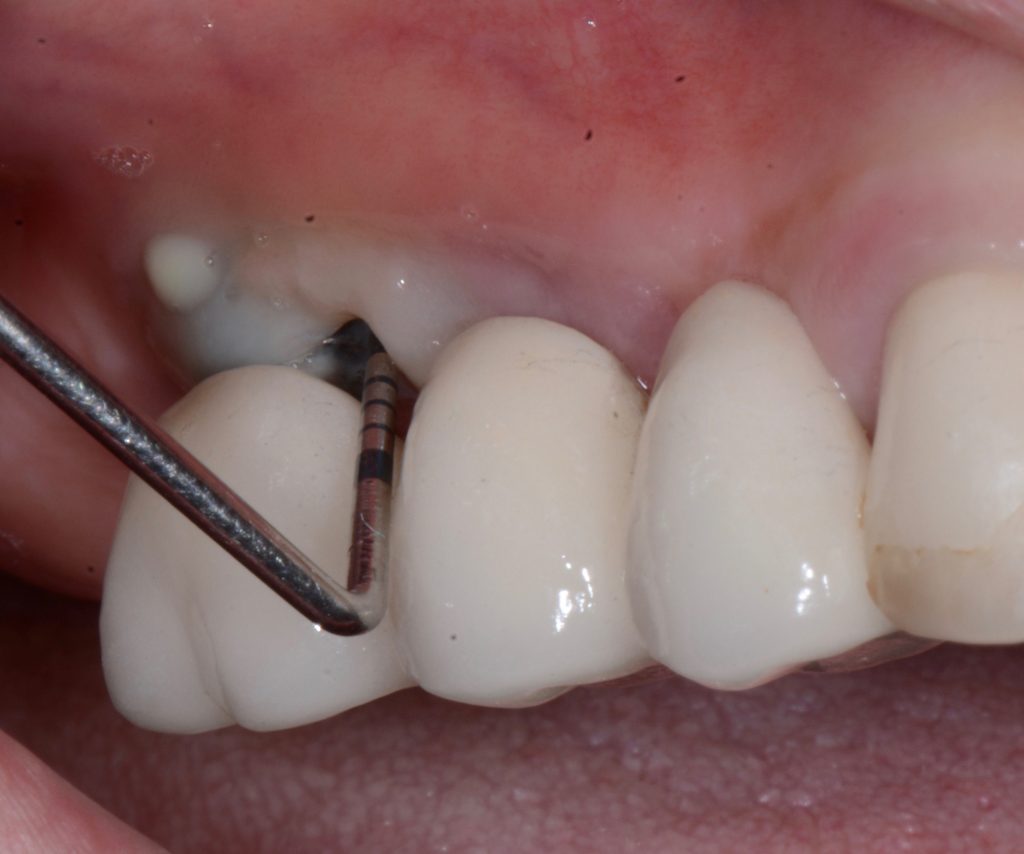
It is mandatory to probe around each implant at 6 points and, using a periodontal probe, to perform a detailed inspection, and, if necessary, additional radiographic examination.
While probing, increased probing depth, as well as presence of BoP and SUP are to be observed. SUP can also be detected by means of palpation.
- The main criterion for the distinction between healthy and inflammatory mucosa is bleeding on probing.
- The main criterion for the distinction between peri-mucositis and peri-implantitis is deterioration in the bone supporting the dental implant.
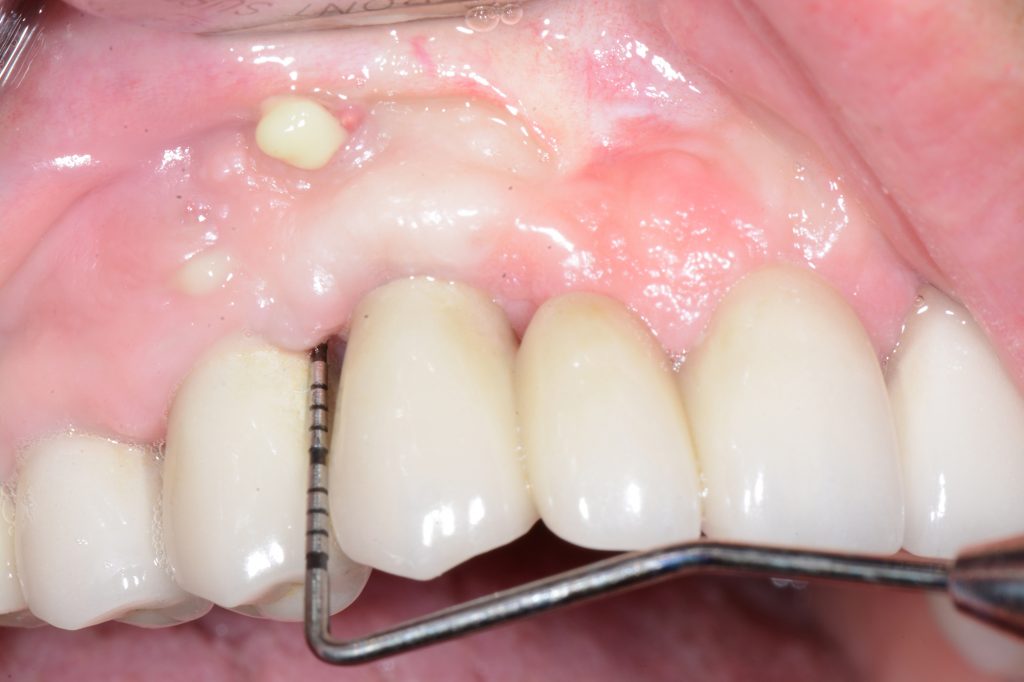
Peri-implant health is characterized by the absence of clinical signs of inflammation in the peri-implant complex. It is outlined as an absence of swelling, redness and BoP. Hence, there is no precisely defined range of probing depths that are compatible with peri-implant health, because peri-implant probing depth depends on the thickness of the peri-implant tissues, type and position of the implant, as well as the type of restoration. In addition, peri-implant health can exist around implants with reduced bony support. It can be established after successful treatment of peri-implantitis.
Major characteristics of implant health:
- absence of clinical signs of inflammation
- absence of bleeding and/or suppuration on gentle probing
- no increase in probing depth compared to previous examinations
- absence of bone loss beyond crestal bone level changes resulting from initial bone remodeling (Berglundh, 2018)
Peri-implant mucositis (PM) can be diagnosed with the first discrete signs of soft tissue inflammation, but without peri-implant bone loss. The common cause is accumulated dental plaque around the implant neck.
The main sign of peri-mucositis is bleeding on mild probing, often accompanied by redness and swelling. Sometimes, there can also be increased probing depth due to swelling. It is important to point out that the main inflammatory process is located exclusively in the epithelial tissue, while the junction between connective tissue and the epithelium is not affected.
Major characteristics of peri-implant mucositis (PM):
- Bleeding and/or suppuration on gentle probing with or without increased probing depth compared to previous examinations
- Absence of bone loss as observed on a radiograph (beyond crestal bone level changes resulting from initial bone remodeling) (Heitz-Mayfield, 2018)
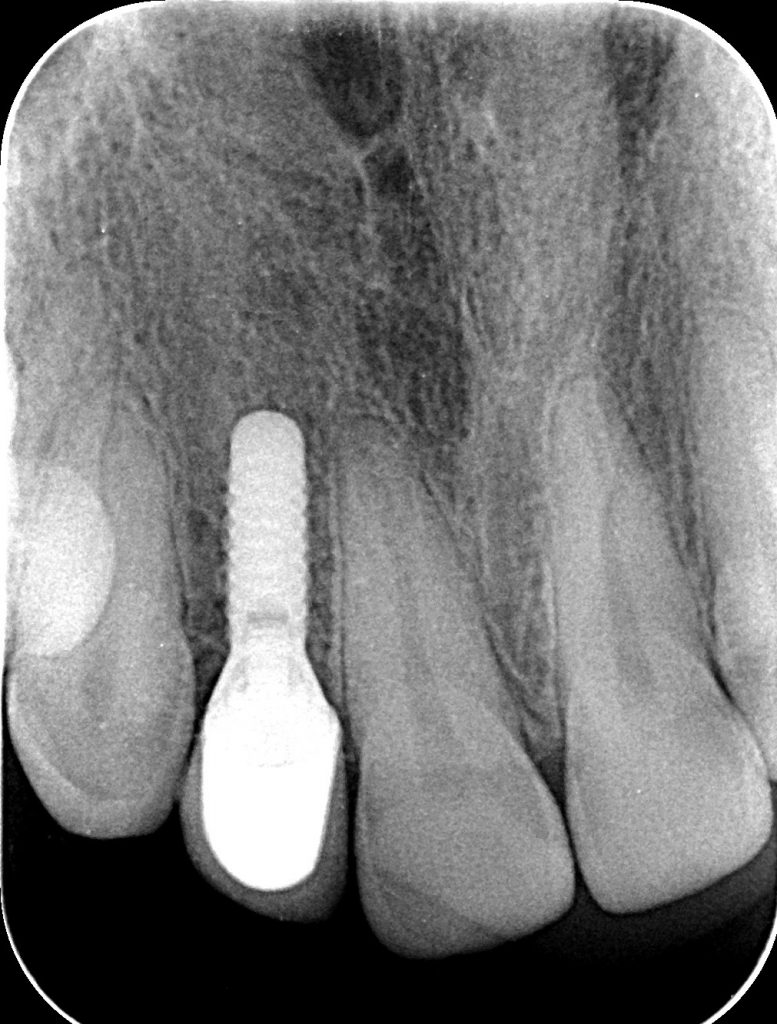
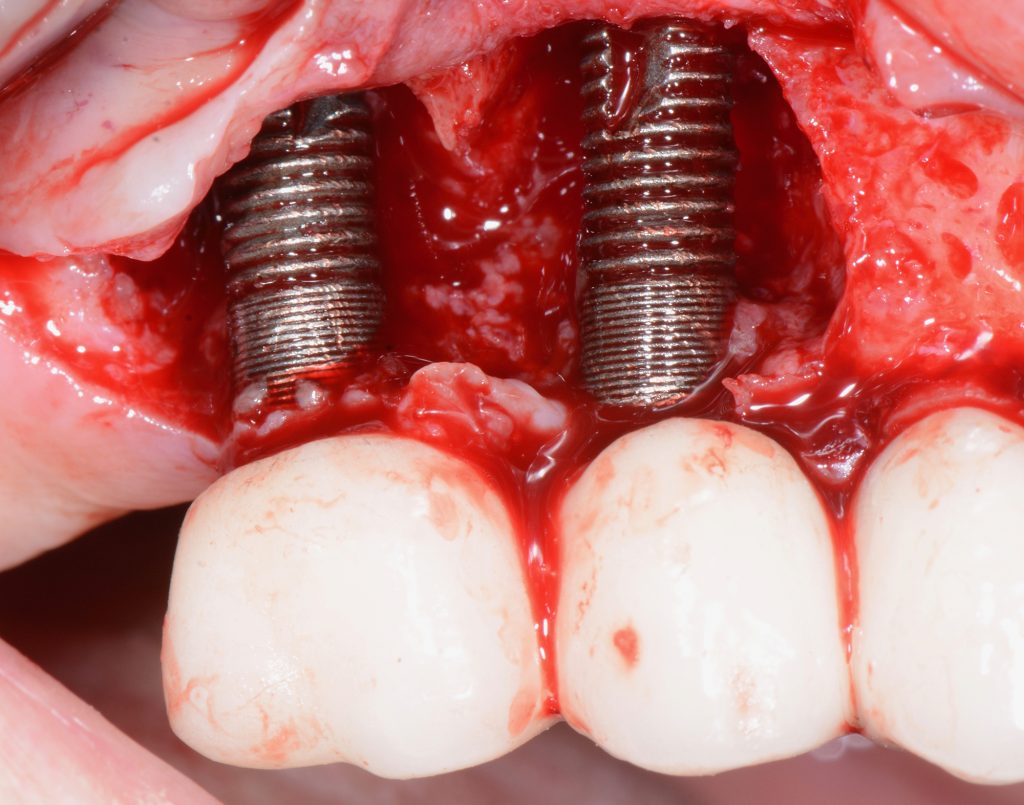
If PM is not recognized and treated during the early phases, the inflammation spreads from the soft tissues into the hard tissues. Apical migration of pathologically changed masticatory mucosa occurs alongside bone resorption. A peri-implant pocket is formed due to bone resorption, and it can be diagnosed by means of probing or by radiographic analysis. The above signs lead to the diagnosis of peri-implantitis (PI). Peri-implantitis is characterized by inflammation of peri-implant soft tissues and progressive bone loss. All clinical signs of inflammation are presented (redness, swelling, increased pain in the area) as well as bleeding and suppuration on probing.
Major characteristics of peri-implantitis (PI):
- presence of bleeding and/or suppuration on gentle probing
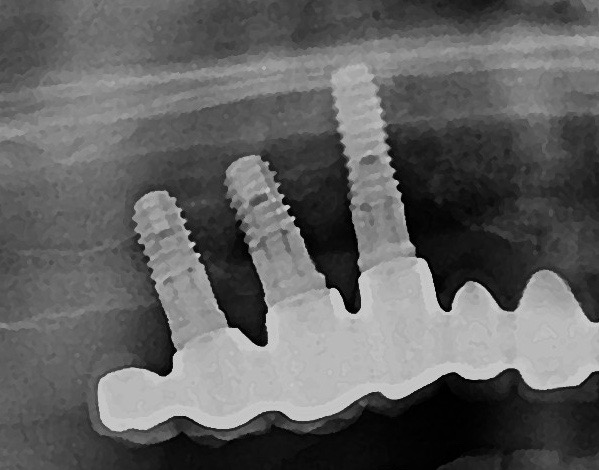
- probing depths of ≥6 mm; bone levels ≥3 mm apical of the most coronal portion of the intraosseous part of the implant (Schwarz, 2018)
Alongside the above-described conditions, terms such as “ailing implant” and “failing implant” can also be seen throughout the literature. There is still no unequivocal definition of implant failure. In other words, clear guidelines don’t exist for when an implant becomes irrational to treat and should be explanted. Misch et al. have summarized general factors defining implant as hopeless. There is still need for further research on this topic.
Implant failure (FI):
- Implant presenting ≥ 50% of bone loss
- Mobile implant
- Loss of osseointegration
- Persistent inflammation described by presence of profuse bleeding and/or suppuration and a radiographically documented continuous bone loss despite treatment (Misch, 2008).
Treatment
The aim of peri-implantitis treatment is to arrest and ultimately eliminate the inflammation by complete removal of the biofilm from poorly accessible implant components and surfaces (i.e., implant threads), as well as to clean the implant (perform surface decontamination) by means of mechanical and/or chemical debridement. The treatment always starts with a non-surgical approach. When a persisting inflammation remains, surgical therapy is indicated.
Non-surgical treatment consists of:
- Mechanical debridement (using special curettes and/or air-polishing devices)
- Local application of antiseptics (e.g., sterile saline, CHX, essential oils)
- Adjunctive antibiotics (local and/or systemic)
- Laser
However, many clinicians still think that without surgical treatment, it is difficult to fully assess all the surfaces and clean the implants and prosthetic components adequately. The surgical approach includes the elevation of mucoperiosteal flaps and thorough debridement of granulation tissue around dental implants, followed by implant surface decontamination. Depending on the defect configuration, either regenerative or resective surgical techniques are indicated. For example, according to the classification based on the defect configuration proposed by Schwarz et al., Class I refers to defects with the presence of an intraosseous compartment, whereas Class II of the proposed classification refers to defects with a horizontal pattern of bone loss. Correspondingly, bony defects around implants are suitable for regeneration when they have an intraosseous/intrabony component. Various regenerative protocols have been proposed with the use of GBR techniques. Soft tissue augmentation techniques have also shown promising/favorable results, given that increased stability of soft tissues and an adequately keratinized tissue width around implants ultimately contribute significantly to long-term results. Furthermore, surgical augmentative peri-implantitis therapy resulted in improved clinical and radiographic treatment outcomes compared with the baseline (CAL, PD and radiographic bone stability) in the majority of studies with 6 months to 7-10 years of follow-up (Khoury, 2019). However, there is no evidence to support the superiority of a specific material, product or membrane in terms of long-term clinical benefits. (Khoury, 2019).
The primary goal of resective peri-implantitis treatment is to reduce soft tissue inflammation and stabilize crestal bone levels, whilst reducing probing depths. Recent data have outlined that the surgical treatment of peri-implant defects yields favorable outcomes in terms of disease resolution (Carcuac et al., 2017). In a resective surgical approach, bone recontouring is traditionally performed in combination with apically repositioned flaps. Moreover, a procedure called “implantoplasty” (implant thread removal and rough implant surface smoothening) may be performed in combination with resective treatment. Implantoplasty is performed at the aspects of the exposed implant where the defect is less amenable to regeneration (suprabony or dehisced areas/zones of the implant). The purpose of this procedure is to reduce the roughness of the exposed threads, thus creating a less plaque-retentive surface to be left exposed in the oral cavity. A recent systematic review found that surgical non-regenerative treatment of peri-implantitis can reduce the amount of inflammation in the short-term follow-up and that the use of implantoplasty may result in the improvement of clinical and radiographic parameters (Keeve, 2019).
When evaluating treatment outcomes, the main factors to be assessed are simultaneous absence of BOP/SUP, PPD £5 mm and bone loss £0.5 mm between the 2-week post-op and follow-up X-rays (Carcuac, 2017).
Multiple studies have demonstrated that surgical treatment yields an improvement in various clinical parameters. Nonetheless, the patient should be informed that mucosal recession can follow surgical therapy, and more importantly, that the disease can progress despite the treatment measures undertaken.
Unfortunately, there is currently no definite consensus on the optimal treatment protocols for peri-implantitis. Various clinical protocols for treating peri-implantitis have been proposed with various degrees of success. In conclusion, it is still for clinicians, depending on their clinical experience and expertise (and following certain predetermined guidelines), to determine the proper treatment protocol and its predictability for each patient individually.
Therefore, all preventive measures should be undertaken to prevent peri-implant complications. Individualized treatment planning, adequate surgical and prosthetic treatment as well as a regular maintenance program are crucial for dental implant success. Since it has been hypothesized that peri-implant mucositis converts to peri-implantitis, early diagnosis and treatment are mandatory. Peri-implantitis treatment should be followed by individualized supportive care (Heitz-Mayfield, 2018). Despite treatment, disease progression can occur. Apart from obvious facts (e.g., implant mobility, loss of osseointegration, implant fracture), a definition of implant failure does not exist. There is still a need for consensus guidelines both on PI treatment protocols and implant failure definitions.