Why to start placing zirconia implants?
“Only if my practice is no longer doing well, will I start placing zirconia implants!” I heard this from a colleague at one of the biggest implant dentistry congresses in Germany and it surprised me. It demonstrates impressively that, even nowadays, the topic “zirconia implants” is still very often discussed in an emotional and even polemic way, rather than in an evidence-based way as should be the case. I think the message sent by this sentence is completely wrong: Placing zirconia implants is not a last-ditch attempt to save a practice that is performing poorly. It is a unique feature and opportunity to enhance and further develop a practice that is performing well! The discussion about placing titanium or zirconia implants must not be considered dogmatically: titanium and zirconia implants can coexist in a serious and reasonable way in our clinical daily routine.
In the last 2 decades, zirconia implants have developed from an initially rather unpredictable and risky treatment modality used only by “mavericks” or “hobbyists” to a scientifically well-documented and very reliable treatment option for serious clinicians. And, by the way, patients like ceramic implants! In a demographic investigation interviewing more than 270 patients in two countries, it was reported that four times more patients would prefer ceramic over metal implants (Gahlert et al. 2018). Thus, it would be a good idea for dentists to give patients asking for ceramic implants sound answers.
Zirconia implant dentistry
The development of high-performance oxide ceramics – like zirconia – has provided new treatment options for clinicians as well as for patients. To establish zirconia as a reliable alternative to titanium as an implant material, fracture-safe zirconia implants with a micro-rough implant surface that show predictable osseous integration had to be developed. At the beginning of 2004, the first 1-piece zirconia dental implants were rolled out. Initially, in terms of material characteristics, creating micro-rough surface topographies on zirconia implants without compromising biomechanical stability (such as fracture toughness and fatigue strength) was a challenging process from a technical point of view. Thus, lower survival rates (Roehling et al. 2018; Morton et al. 2018) and numerous zirconia implant fractures were reported for the “first generation” of zirconia implants (Roehling et al. 2016; Gahlert et al. 2013; Gahlert et al. 2012). Since then, the industry has constantly improved manufacturing processes to produce micro-roughened zirconia implants with reliable fracture toughness and fatigue strength. In this context, not only surface micro-texture but also implant macro design have been adapted. Whereas the first zirconia implant systems were limited to a 1-piece design, 2-piece zirconia implants have also become available on the market in the meantime. This progress has also been strongly influenced by the predilection of clinicians for 2-piece implant designs. Today, zirconia implants with different designs and diameters that allow the treatment of completely and partially edentulous patients are available on the market.
Zirconia material characteristics compared with titanium and other metals
Zirconium dioxide (zirconia, ZrO2) is an oxide ceramic that is composed of the single elements zirconium and oxygen which are both firmly interconnected and stabilized in a specific crystal lattice. This means that the oxygen element is a key part of the bulk zirconia material structure itself (Fig. 1). In contrast, the titanium bulk material does present mainly the titanium element alone. Every titanium atom is locked in a specific crystal lattice. Only at the surface, a very thin oxide layer of a few nanometers is automatically and immediately formed on the titanium implant surface when exposed to air if the surface atmosphere is not controlled and/or protected (Kasemo 1983; Roehling et al. 2015). Even if this layer is made of titanium dioxide, it does not give any physical ceramic properties to titanium. However, it ensures there are no unwanted reactions between the titanium and the surrounding biological soft and hard tissues. Thus, titanium is not a “bioinert material” but has a “bioinert character” by the formation of the stable oxide layer on its surface.

Misleadingly, zirconia oxide ceramics are very often labeled “zirconium”. “Zirconium” is a pure metal and “zirconia” refers to the “zirconium dioxide”. As with titanium, the zirconium element can be found in the 4th group of the periodic system. Thus, zirconia oxide ceramics have to be distinguished from the metal element zirconium and from zirconium metal alloys. From a chemical point of view, a characteristic feature of metal (for example titanium alone) and metal alloys (for example the known dental titanium-zirconium alloy: Roxolid®) is the so called “metallic bond” that firmly connects the single metal elements of the alloy and shares moving electrons: the electrons are delocalized in the structure forming a cloud. In contrast to that, the single elements of oxide ceramics are not connected with a metallic but with a so-called “ionic bond”: the electrons are locked and localized. Therefore, electrons cannot be released from the material composition and result in unwelcome reactions – such as corrosion – with the surrounding soft and hard tissues (Roehling & Gahlert 2017).
Zirconia – Fracture toughness
In comparison to other oxide ceramics (e. g. alumina), zirconia shows superior biomechanical properties such as high fracture toughness and bending strength (Christel et al. 1989) that give zirconia implants the ability to withstand oral occlusal forces (Andreiotelli et al. 2009; Silva et al. 2009). In this context, the so-called “phase transformation” – describing the formation of a fracture-proof crystal phase (tetragonal crystal structure) to a less fracture-proof crystal phase (monoclinic crystal structure) – of zirconia is very important (Roehling & Gahlert 2015). This transformation is associated with a volume expansion of the zirconia crystals that can stop mechanically-induced micro-cracks in the material structure (transformation toughening mechanism) (Roehling & Gahlert 2015). Wrong treatment or processing of the zirconia material (such as uncontrolled grinding procedures of the implant body, non-optimized manufacturing processes) can induce unwanted, premature phase transformation. Consequently, mechanically induced micro-crack propagation in the crystal structure can only be compensated to a limited extent. It can be hindered to some degree until the point that the phase transformation from tetragonal to monoclinic has finalized around the cracks. Thus, manufacturing and handling procedures cannot simply be transferred from titanium to zirconia since the material structure of zirconia can be damaged by uncontrolled techniques. This fact has to be taken into consideration by manufacturers as well as clinicians (Roehling & Gahlert 2017).
In recent years, manufacturing processes and particularly the methods for creating micro-rough implant surfaces have been improved and adapted to the material characteristics of zirconia. Interestingly, a meta-analysis has reported that the fracture incidence of zirconia dental implants significantly improved from 3.4% to 0.2% between 2004 and 2017 (Roehling et al. 2018). Based on these facts, implant fractures are no longer a clinically relevant problem for the latest generation of zirconia implants.
Aging of zirconia implants
In a wet environment – such as the oral cavity – phase transformation can occur without mechanically-induced micro-cracks. In this context, the slow transformation from tetragonal to monoclinic is called “aging”, also referred to as “low temperature degradation”. Scientific studies have shown that a longer aging period is directly associated with an increased degree of phase transformation. However, a considerable amount of phase transformation did not lead to a decrease in fatigue strength and fracture toughness (Sanon et al. 2013; Chevalier et al. 2011; Monzavi et al. 2020). Consequently it can be concluded that aging and low temperature degradation do not have any clinically relevant effect on the biomechanical long-term stability of zirconia dental implants.
Zirconia implants – hard tissue integration
The safe and reliable biological stabilization of the implant in bone tissue – also referred to as osseointegration – is the most important precondition for the clinical success of an implant. For titanium implants, the development of micro-rough implant surfaces was the key factor for reliable osseointegration and the establishment of titanium implants on the market (Buser et al. 2012, Cochran et al. 2011, Roehling et al. 2015). A comparable evolution of surface technologies has been observed for zirconia implants. The first generation of zirconia implants had rather smooth implant surfaces and consequently showed reduced implant survival rates compared with micro-rough titanium implants (Roehling et al. 2014). Later on, improved manufacturing processes allowed the creation of micro-rough surface topographies on zirconia implants. Consequently, the osseointegrative capacity of zirconia implants has been improved (Roehling et al. 2019). Experimental studies have shown that the latest generation of zirconia implants with micro-rough surfaces show the same hard tissue integration as titanium implants (Bormann et al. 2012; Gahlert et al. 2012; Gahlert et al. 2009; Gahlert et al. 2010; Janner et al. 2018; Roehling et al. 2019). The latest generation of zirconia implant surface topography – which is created by mild sandblasting followed by an acid-etching process – is comparable to the sandblasted and acid-etched surface of titanium implants (Fig. 2).

The zirconia implant systems available on the market, however, show varying surface topographies and not every company offers evidence-based data or provides information regarding the implant surface and osseointegrative behavior of their product. Consequently, clinicians must check if the zirconia implant system used offers scientific data regarding its osseointegrative capacity.
Zirconia implants – soft tissue integration
Besides the natural appearance of the crown (white esthetics), pink esthetics – characterized by un-irritated peri-implant soft tissue conditions, healthy soft tissue margins and papilla formation – are important to treatment outcomes. From a periodontal point of view, gingival tissues around implants have a similar barrier function to dento-gingival tissues around teeth to help prevent bacterial peri-implant diseases (Berglundh et al. 1991). For pink esthetics, healthy and constant vertical peri-implant soft tissue dimensions are important. Around implants as well as teeth, the soft tissue is composed of the sulcular epithelium, sulcus depth, junctional epithelium and connective tissue contact. Taken together, these form a vertical unit, the so-called biologic width.
Experimental studies have shown a similar qualitative and quantitative soft tissue integration and the same biologic width dimensions around zirconia and titanium implants (Roehling et al. 2019; Roehling & Cochran 2018). Thus, the implant material did not significantly influence soft tissue integration. Consequently, similar physiological processes regarding soft tissue formation are supposed for both materials (Roehling et al. 2019). However, faster maturation processes of the peri-implant epithelial and connective tissue have been assumed for zirconia implants (Linares 2016).
Interestingly, for zirconia as well as for titanium implants, biologic width dimensions and peri-implant papilla height are not dependent on the loading or surgical protocol but on the implant design and the vertical position of the micro-gap between implant shoulder and prosthetic supra-structure (Roehling et al. 2019; Roehling & Cochran 2018). In addition, clinical studies have reported a significant increase in peri-implant papilla height over time. In this context, the distance between the alveolar crest at the neighboring tooth and the lowest point of the contact area of the crown appears to be an important factor for interdental papilla formation (Kniha et al. 2016; Roehling & Cochran 2018).
Zirconia implants – peri-implant infections
The etiology of peri-implant infections is multifactorial, while microbial colonization on implant surfaces is considered as one of the main factors (Mombelli 2994). Results from experimental studies have reported promising results for zirconia. Significantly reduced biofilm formation has been shown for zirconia compared with titanium after an incubation period of 72 hours (Roehling et al. 2017). Moreover, an experimental study in canines investigated ligature-induced peri-implant infections around loaded zirconia and titanium implants. After an active and spontaneous progression period, zirconia implants revealed significantly less peri-implant bone loss. Interestingly, one titanium implant was lost during the study; however, no zirconia implant failures occurred (Roehling et al. 2018). From a clinical point of view, only very little data is available regarding peri-implantitis around zirconia implants. Only one clinical study reported an “initial peri-implantitis” around 18 out of 48 evaluated zirconia implants 12 and 24 months after implant placement (Becker et al. 2017). In contrast, a retrospective clinical study investigating 161 zirconia implants did not report any signs of infection after a functional loading period up to and after 7 years. The results demonstrated that the observed early and late implant failures were not associated with any clinical signs of infections (Roehling et al. 2016). Based on the available evidence, it is hardly possible to say whether zirconia implants have a minor susceptibility to the formation of peri-implant infections. However, the results that have been reported so far are promising.
Zirconia implants – clinical data
In the last couple of years, various clinical studies investigating different types of zirconia implants were published. However, it must be considered that some studies investigated zirconia implant systems that have been further developed in the meantime and are no longer available on the market. A meta-analysis has reported that physical properties and ongoing market availability significantly influenced the reported zirconia implant survival rates (Roehling et al. 2018). In a systematic review, the author evaluated clinical studies investigating zirconia implants that were published between 2004 and 2017. The reported 1-year mean survival rate for commercially available (CA) zirconia implants (98.3%) was significantly higher compared with zirconia implants that are no longer commercially available (NCA) on the market (91.2%). In contrast, the differences between both groups were not statistically significant regarding peri-implant bone loss after 1 year. In addition, the meta-analyses evaluated a mean 2-year survival rate for CA zirconia implants (97.2%) whereas factors like implant design, loading and implant placement protocol, simultaneous bone augmentation procedures or type of prosthetic reconstruction did not significantly influence the reported survival rates. For the first time, it has been shown that zirconia implant survival rates significantly increased between 2004 and 2017 (Roehling et al. 2018). However, it must be noted that not all zirconia implant systems available on the market offer evidence-based clinical data.
Even though meta-analyses estimating overall survival rates are currently limited to 1- and 2-years data, single studies reported longer clinical follow-up periods. Regarding commercially available zirconia implants, clinical data up to and beyond 5 years of functional loading, reporting survival rates of 95% and more are available (Gahlert et al. 2016; Grassi et al. 2015; Bormann et al. 2018). Most recently, a prospective clinical study investigated 71 zirconia implants placed in 60 patients. After a mean functional loading of 5.6 years, the authors reported a survival rate of 98.4% and a mean peri-implant bone loss of 0.7 mm (Balmer et al. 2020).
Besides the lower amount of clinical data available with zirconia implants in comparison with the available data reporting on titanium implants, the published results show a similar level of survival rates which validate the clinical usage of micro-rough zirconia implants.
1-piece zirconia implants
Based on the monotype design – meaning that the implant abutment is an inherent part of the implant body – there are only limited possibilities to correct the implant axis with the prosthetic supra-structure. Therefore, adequate backward planning and prosthetically driven implant placement are extremely important. Moreover, since the abutment extends into the oral cavity, implant overloading during the early healing period – if immediate loading is not wanted – has to be avoided. Monotype implants are always used with cement-retained prosthetic reconstructions. Thus, defined protocols for the cementation process are necessary to avoid cement remnants in the peri-implant mucosa (Fig. 4). 1-piece zirconia implants can be fully integrated into the digital workflow. Zirconia implants with a 1-piece implant design are very often considered an “old-fashioned” treatment modality or a “niche product” since it is not possible to fabricate individual abutments. However, we have to keep in mind that by using monotype implant designs, problems due to micro-leakage or micro-gaps can be avoided. If 1-piece implants are placed correctly, very good, predictable and reproducible esthetic outcomes can be obtained. According to my more than 10 years clinical experience, the most beautiful outcomes regarding white and pink esthetics are achieved by using monotype zirconia implants (Fig. 5).
2-piece zirconia implants
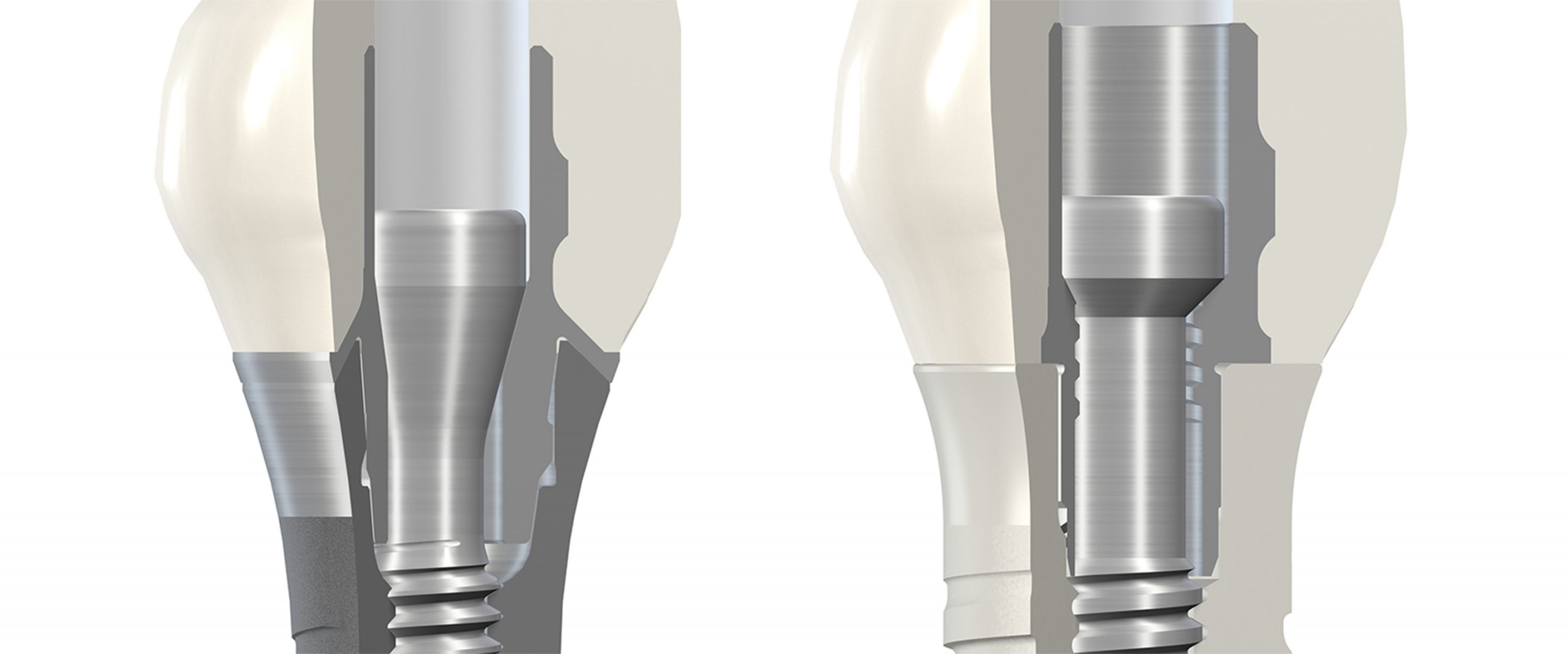
In recent decades, placing titanium implants with a 2-piece design has become a routine procedure for clinicians. Consequently, to make zirconia implants attractive to a larger number of clinicians, zirconia implants with a 2-piece implant design had to be established on the market. Compared with monotype implants, 2-piece zirconia implant designs offer expanded possibilities for the correction of the implant axis, since individual abutments can be fabricated. In addition, the surgical and prosthetic handling is more familiar compared with the clinical application of titanium implants. Regarding the prosthetic connection, 2-piece designs are available that either require cementation of the abutment or that allow a reversible screw-retained connection of the prosthetic supra-structure to the implant body. Thus, only if the 2-piece design allows, reversible screw-retained prosthetic connections are possible and intraoral cementation processes can be avoided (Fig. 6). 2-piece zirconia implants can also be fully integrated in the digital workflow. Currently, no 2-piece zirconia implants with reduced diameters (less than 4 mm) are available on the market.
2-piece zirconia implants – reliable screw-retained prosthetic connections?
Certainly, 2-piece zirconia implants are necessary to sustainably establish ceramic implants on the market. However, 2-piece zirconia implant designs make high demands on the manufacturing process. In contrast to 1-piece zirconia implant designs, not only a micro-rough surface topography, but also the implant inner design has to be fabricated and adapted to zirconia’s material properties without damaging the fracture and fatigue strength of the implants. Compared with titanium, zirconia is a brittle material with a higher microhardness and a higher Young´s modulus. Thus, processing zirconia is quite complex, and the shear strength is very important in terms of fracture toughness. This fact has to be considered regarding the prosthetic connections of 2-piece zirconia implants. A stable and reliable connection between the implant body and prosthetic supra-structure can be guaranteed with mainly 2 different mechanisms: the firm connection is mostly achieved either by the retention screw or by the inner design of the implant (e.g. high friction with a conical connection). If the main function of the retention screw is to guarantee a stable connection between implant body and supra-structure, it is not currently possible to use zirconia screws to connect the abutment with the implant body. Consequently, other materials than zirconia like titanium alloy or carbon-reinforced PEEK screws are offered to reversibly connect the prosthetic supra-structure to the implants. If the main retention of the suprastructure is not to be achieved by the screw but with a conical design of the internal connection of the implant and abutment, ceramic screws might be an option where a very reduced strength is then necessary. In the future, new or further optimized manufacturing and sintering processes will enhance the biomechanical properties of zirconia and might allow new possibilities for full ceramic screw-retentions.
In the last couple of years, a 2-piece zirconia implant with a titanium retention screw has proven its clinical reliability. This implant type makes it necessary for a ceramic supra-structure to be bonded to a titanium abutment which is fixed to the ceramic implant with a titanium screw. To guarantee a stable connection between the titanium screw and the full ceramic implant, the inner design was adapted to the zirconia material properties. This is a very important fact since zirconia is 5 – 9 times harder than titanium: if the design of the internal configuration of the implant contains sharp-edged features, particles from an interacting metal abutment can be shaved off, which in the worst case could lead to wear and screw loosening. Consequently, wear behavior is mainly influenced by the design and the manufacturing process and not just by the material components. For titanium implants, evidence-based data has shown that technical complications like screw loosening are mainly influenced by the design of the implant abutment connection, anti-rotational features and screw preloading torques (Theoharidou et al. 2008; Pjetursson et al. 2018).
Compared with a typical titanium implant, the design of the internal configuration of the zirconia implant has been adapted to the material properties of zirconia in order to reduce the mechanical stress on the implant components (Figs 7 – 8):
- The internal square geometry in the implant has been extended
- Edges and chamfers at the contact surface and the interface of the implant have been replaced by radial and tangential transition zones (design in line with materials)
- The surface of the internal square has been refined by employing an enhanced production process (reduced friction coefficient)
- The external square geometry of the titanium base has been extended and optimized to ensure precision fit in the implant (reduced rotational play and tilting)
- The fit of the titanium screw has been optimized and the contact surface angulation has been raised in order to increase the screw pretension
- The connection presents a flat seating for the abutment. Since the implant shoulder is wide enough (diameter 4.8 mm), it leaves enough space to avoid a conical connection and to propose the mentioned flat top connection, which is optimal for ceramics in terms of mechanical properties and for a tissue level implant type
Most recently, the stability of this titanium-zirconia screw-retained connection has been directly compared with a conventional titanium-based connection in an in-vitro chewing simulation including artificial aging. Incisor and molar-shaped monolithic zirconia crowns were screw-retained on either 2-piece zirconia or 2-piece titanium implants. The implants were subjected to artificial aging by thermal cycling and mechanical loading of 1.2 million chewing cycles (simulating 5 years of functional loading). In addition to that, the implants were also subjected to a fracture force test to evaluate fracture toughness. The results have shown no statistically significant differences between the investigated groups. Consequently, the authors reported “The connection of the tested screw-retained zirconia crowns in two-piece zirconia implants is comparable to standard titanium implants in the specific in vitro testing” and “Based on the results of the present study, the connection between crown and the two-piece zirconia implant seems to be suitable for clinical application“ (Joos et al. 2020).
Summary
Zirconia implants have been established on the market since 2004. While the first zirconia implant systems were limited to a 1-piece design, 2-piece zirconia implants have also become available on the market in the meantime. Optimized manufacturing processes and implant designs allow the fabrication of fracture-proof 1- and 2-piece zirconia implants with micro-rough surfaces. Whether the biocompatibility of zirconia offers advantages regarding the formation of peri-implant infections has to be investigated in future studies. However, first results from experimental studies are promising. Scientific investigations have reported that micro-rough zirconia implants show identical soft and hard tissue integration and similar clinical survival rates compared with titanium implants. However, more clinical long-term data is needed. 1- and 2-piece zirconia implants of the newest generation that offer evidence-based data are a reliable alternative to established titanium implants and offer a safe and serious expansion of the clinical daily routine.
Learn more: