Introduction
Implant dentistry has developed extensively since the first application of dental implants by Dr. Brånemark during the 1960s. In today’s modern dentistry, more and more patients are turning to dental implants to replace their missing teeth with high expectations of a pleasing outcome that goes beyond the functional success of osteointegration. Therefore, in addition to hard tissue augmentation, soft tissue management around dental implants has become a hot topic that is attracting a lot of attention from the dental implant community. The stability of soft tissue around dental implants has become an essential and key element towards achieving function, esthetics, and long-term maintenance of dental implants.
Understanding the characteristics of the periodontium around natural teeth and peri-implant soft tissue –similarities and differences
The structure and function of the periodontium around natural dentition is determined by the integration of four main tissues: the periodontal ligament (PDL), tooth root cementum, alveolar bone, and gingiva. The presence of Sharpey’s fibers, which are the terminal ends of principal fibers (of the periodontal ligament) that insert into the cementum and the periosteum of the alveolar bone, provides a tight connection between the natural teeth and surrounding tissue. When we look at the gingival tissue, the epithelium covering the free gingiva can be differentiated into the oral epithelium(OE), oralsulcular epithelium (OSE) and junctional epithelium(JE). In the classical periodontal literature back in the 1960s, Dr. Schroeder stated that at the most apical position closest to the cementoenamel junction (CEJ), the JE comprises only 3-4 layers of cells in normal periodontal tissues. The cells attached to the tooth surface by hemidesmosomes show a high metabolic rate and the presence of immune cells indicate this is the host’s first line of defense against bacterial invasion. Collectively, the structure of the periodontium around natural dentition provides a biologic and physical barrier to multiple challenges that the teeth sustain as a result of the occlusal function and the challenging microbial environment of the oral cavity (Schroeder 1966,1986).
One of the main differences between dental implants and natural dentition is the integration of the implant fixture and the surrounding alveolar bone, which was discovered by Professor Per-Ingvar Brånemark back in 1969, so-called osteointegration. Therefore, there is no periodontal ligament between the dental implant and the alveolar process. However, if we take a look at the soft tissue interface between the dental implant and the surrounding gingival tissue, the current scientific literature indicates there is a structural similarity comparable to dentogingival junctions around natural dentition, including the presence of the junctional epithelial attachment and sulcular.
There is also a peri-implant connective tissue attachment. In natural teeth, the gingival fibers run perpendicular to the tooth’s long axis, attach to and sometimes penetrate the tooth’s structure. With implants, however, the fibers run parallel to the implant’s long axis and do not penetrate the implant’s surface. In addition, the natural tooth has nine different types of supracrestal fibers that enhance attachment while the dental implant has two at most. Therefore, the connective tissue adhesion with implants may have less mechanical resistance compared with natural teeth (Gould et al. 1981, 1984; Jansen et al. 1985; Moon et al. 1999).
When compared to natural dentition, it was found that implants exhibit a poor gingival vascular blood supply. The vascular supply to the dental gingiva derives from the main sources including supraperiosteal blood vessels and the vascular plexus of the periodontal ligament. However, Berglundh et al. (1994) observed that the vascular system of the peri‐implant mucosa originated solely from the large supraperiosteal blood vessel on the outer surface of the alveolar ridge.
In conclusion, the gingiva around natural teeth and dental implants share certain characteristics, but differ in the composition of the connective tissue, the alignment of the collagen fiber bundles, and the distribution of vascular structures in the compartment apical to the barrier epithelium.
How does peri-implant soft tissue play its important role?
Several authors supported the idea of keratinized mucosa (KM) around implants, while others felt it is not a necessity. (Albrektssonet al. 1986; Strubet al. 1991). In an animal study, Warrer and coworkers (Warreret al. 1995) examined the influence of plaque accumulation on attachment loss and recession around implants placed in areas lacking KM. They concluded that implants without KM exhibited more recession and attachment loss compared to those with KM. The lack of KM around implants increases the susceptibility of the peri-implant site to more destruction.
On the other side, Wennstrom (Wennstrom et al. 1994) performed a human study evaluating the soft tissue conditions around dental implants in relation to the width of masticatory mucosa with the opposite results. The study failed to support the concept that the lack of an attached portion of masticatory mucosa may jeopardize the maintenance of soft tissue health around dental implants. However, recent review articles (Lin et al. 2013; Gobbato et al. 2013) indicate the association between inadequate width of keratinized gingiva (KG) (<2 mm) with peri-implant mucositis, which is more prone in the group of patients with erratic maintenance compliance (Monje and Blasi 2019).
In 2020, Gustavo Avila-Ortiz and coworkers further proposed the idea of the peri-implant phenotype, which encompasses a soft tissue component constituted by the peri-implant keratinized mucosa width (KMW), the mucosa thickness (MT) and the supracrestal tissue height (STH), as well as an osseous component, characterized by the peri-implant bone thickness (PBT). They concluded research is necessary to determine the minimum amount of KMW, MT, STH, and PBT required to obtain optimal short- and long-term outcomes, including maintenance of peri-implant health, function and esthetics in specific clinical scenarios.
The surgical technique of soft tissue management around dental implants
A number of surgical techniques can be utilized for gingival augmentation. The earliest of these techniques are the “vestibular extension operations”. The concept was mainly designed to extend the depth of the vestibular sulcus (Bohannan 1962) and in consequence the newly generated tissue covers the exposed periosteum or bony surface. Recently, pedicle or free soft tissue grafts have become the most commonly used techniques to manage “insufficient” gingival dimensions, mainly because of the higher predictability of the treatment result. A pedicle graft is defined as a flap that is left attached to the original site by a narrow base of tissue to provide a blood supply during the grafting procedure, such as the laterally sliding flap and the coronally repositioned flap. On the other hand, in free soft grafts the tissue is freed from its bed and completely removed – after transplantation the graft body is completely separated from the original donor site. The most common free soft graft procedure is the free gingival graft or subepithelial connective tissue graft.
Free Gingival Graft
Bjorn (1963) first described the use of free gingival grafts in periodontal therapy, and Sullivan and Atkins (1968) further discussed the principles of successful grafting. First, the recipient site must be able to form “capillary outgrowths” to vascularize the graft. Adequate hemostasis before grafting is also essential to prevent hematomas and dead space under the graft. Secondly, donor tissues are usually taken from the maxillary palatal region lingual to the bicuspids and molars but they could also be harvested from the edentulous ridge, attached gingiva or maxillary tuberosity. Scarred, fatty, or glandular tissues should be removed to avoid the possibility of vascularization blockage. The third key point is immobilization of the graft. It is necessary to enhance and maintain capillary ingrowths during the healing process. Last, the graft thickness also plays an important role in the outcome of the procedure, which will dictate its contraction, survival, and esthetic result. Primary contraction occurs immediately after the graft is taken from the donor area, and secondary contraction takes place during the stage where the graft integrates into the recipient bed. Thicker grafts have greater primary contraction due to the greater amount of elastic lamina propria but demonstrate less secondary contraction. These are more stable after integration with a rigid recipient bed. Once integrated, thicker grafts are better able to withstand functional stresses. Currently, the free gingival graft (FGG) is considered one of the standard approaches for soft tissue augmentation around dental implants. The following clinical case demonstrates step by step how to use FGG to increase the keratinized gingival tissue around dental implants.
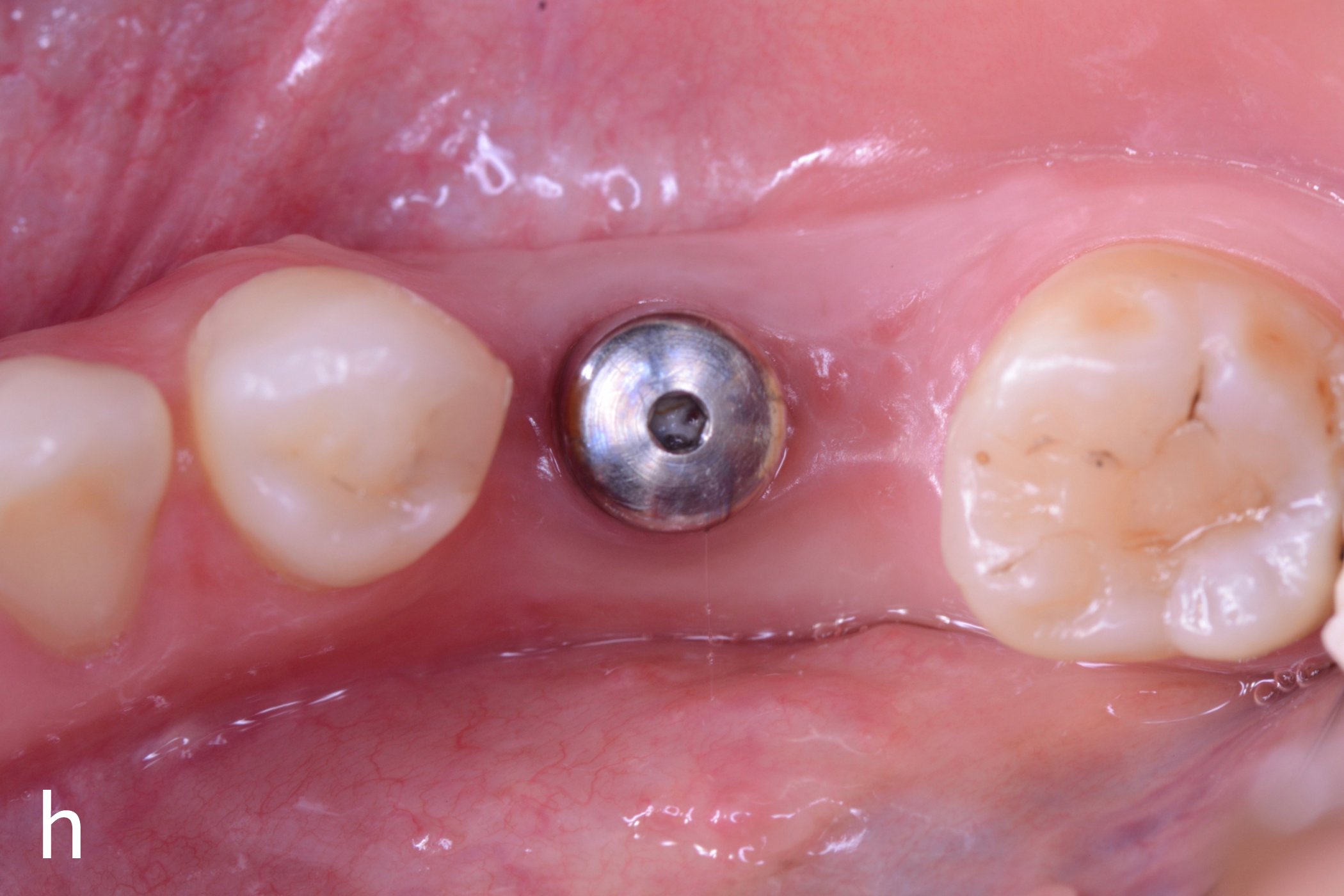
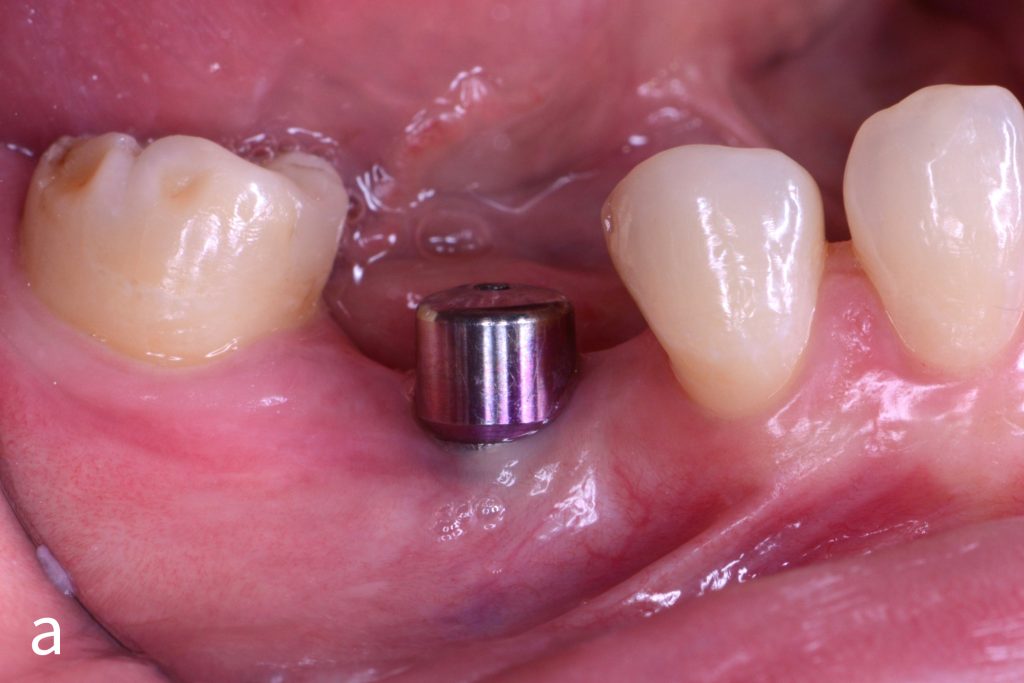
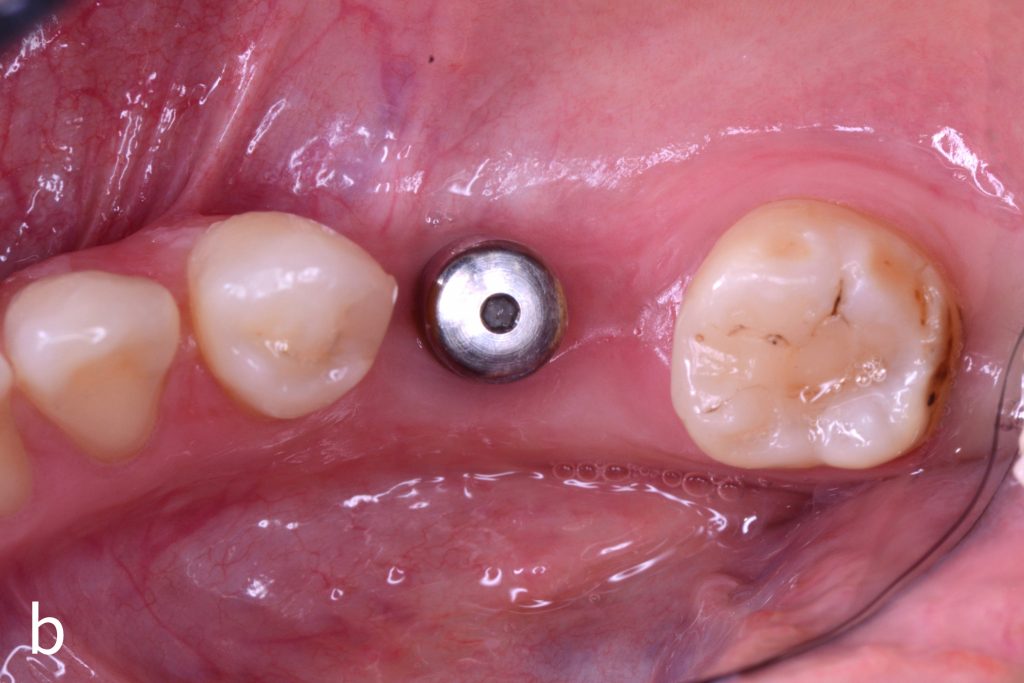
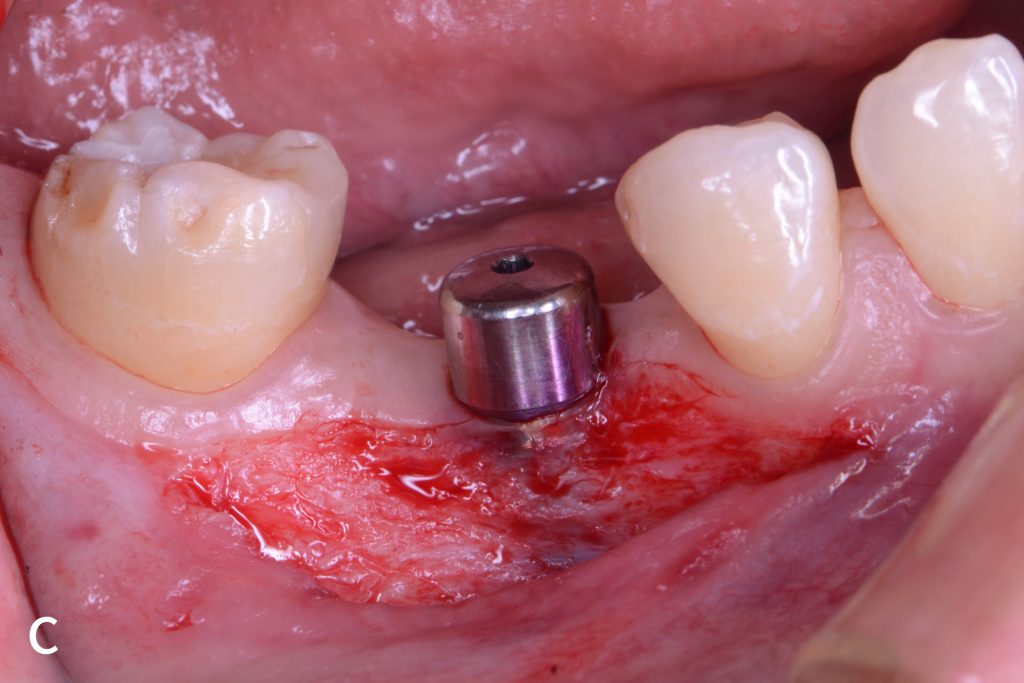
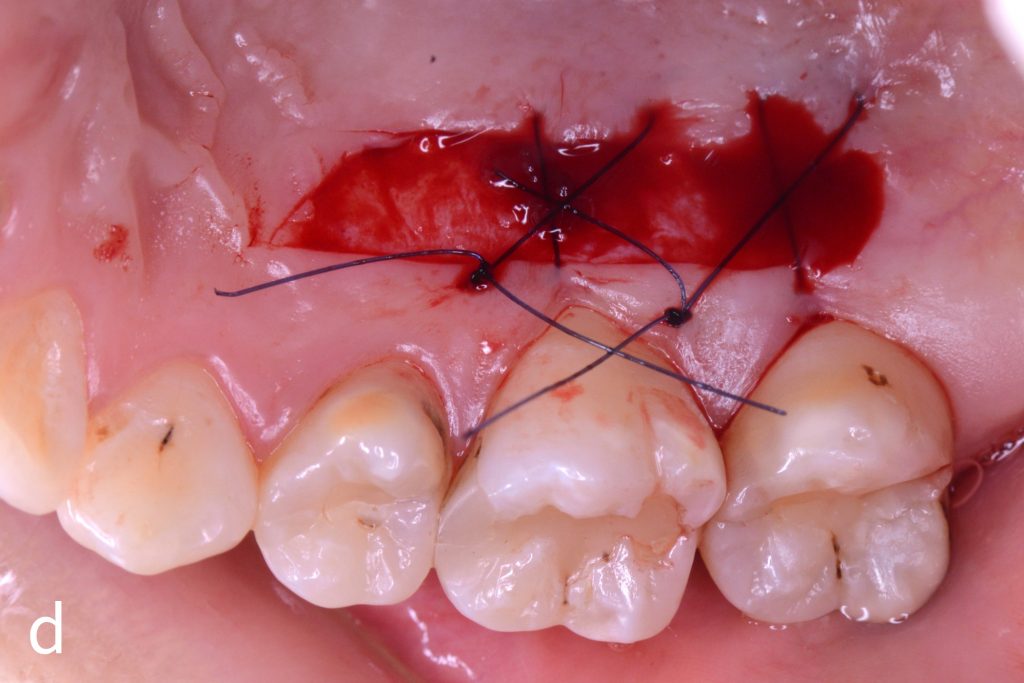
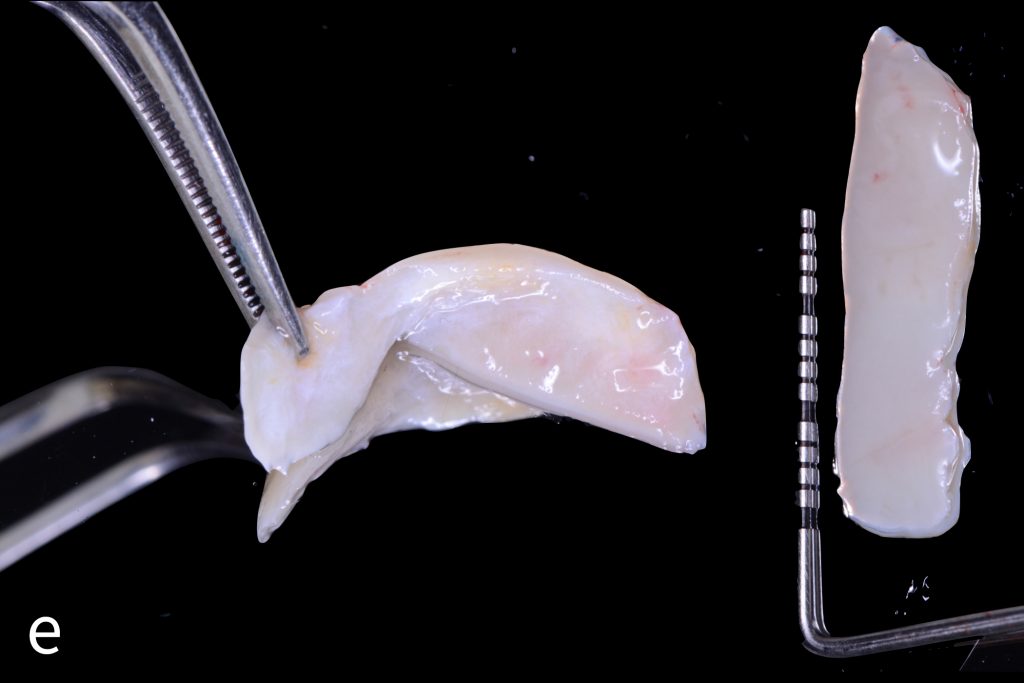
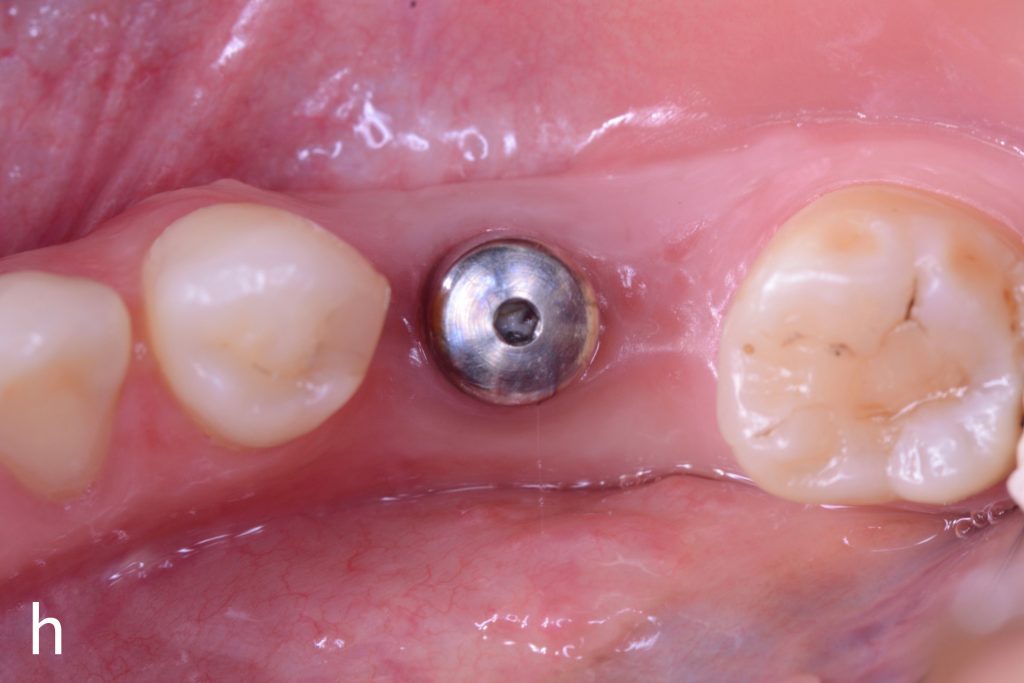
Figs 1a – h: Free gingival graft procedure around a dental implant
The patient presented with an osteointegrated dental implant at the mandibular right first molar. The implant had been placed two years previously and felt sensitive around the gum tissue after delivery of the final prosthesis. Upon clinical examination, the lack of keratinized gingiva at the buccal side the dental implant was noted and also that the implant surface was covered by only a thin layer of mucosal tissue, exposing the metal color (Figs 1a – b). Therefore, a free gingival graft was recommended to increase the amount of keratinized gingiva and soft tissue thickness. On the day of surgery, after local anesthesia, a partial thickness flap was raised at the recipient area of the mandibular right first molar to prepare the recipient bed. A thin layer of the buccal bone plate can be observed (Fig. 1c). An epithelialized graft was harvested from the patient’s right palate and then the recipient bed was stabilized (Figs 1d – e). Cross-link and simple interrupted sutures were placed for tight tissue adaptation to ensure graft stability (Fig. 3f). Four months after surgery, the gingival thickness and the width of keratinized tissue was successfully increased (Figs 1g – h).
Subepithelial connective tissue graft
The application of free gingival grafts has a long history with promising postoperative results. However, there are several limitations to this procedure: (1) It is challenging to increase a significant amount of soft tissue when the thickness is severely limited (2) The esthetic concern after healing of the epithelized graft. The graft does not blend in easily with the surrounding soft tissue and the surface texture, and the color will often be dissimilar, also scar tissue may form with relative ease.
In 1975, Drs. Thorkild Karring, Niklaus P. Lang & Harald Löe introduced the use of the subepithelial connective tissue graft. This experiment plays an important role as a breakthrough in the field of periodontal plastic surgery. In this animal study, subepithelial connective tissue harvested from beneath KG of the hard palate were transplanted into non-keratinized mucosa in the test group. At the end of the study, gingival connective tissue grafts were covered with keratinized epithelium, displaying the same characteristics as those of normal gingival epithelium, while in the control group the alveolar mucosa transplants were covered with non-keratinized epithelium. This indicated that gingival connective tissue regulates the differentiation of the overlying epithelium and is capable of inducing the formation of a keratinized gingival epithelium.
The application of the subepithelial connective tissue graft shows its potential to provide excellent esthetic results and a high success rate, and it further advances the development of the field of periodontal plastic and microsurgery. Langer and Langer, in their 1985 publication, described the step-by-step procedure of the sub-epithelial free connective tissue graft for obtaining dental root coverage. The authors stated that the use of the graft enhances esthetics through color match and also by avoiding the “keloid” healing appearance with free gingival grafts.
Peter B. Raetzke in 1985 described a variation of the traditional sub-epithelial connective tissue graft by minimizing recipient site exposure. The author called it the “envelope” technique where instead of making two vertical releasing incisions to facilitate graft placement, an envelope pouch was created by undermining partial thickness incisions around the denuded root surface. For the treatment of multiple recession sites, a variation of techniques including the tunnel technique proposed by Allen (1994), Bruno (1994), Zabalegui (1999) and the VISTA technique of Dr. Homa Zadeh (2011) showed the trend of a minimally invasive and microsurgical approach in modern mucogingival plastic surgery. The following clinical case demonstrates step by step how to use a subepithelial connective tissue graft to increase the gingival thickness around dental implants in the esthetic zone.
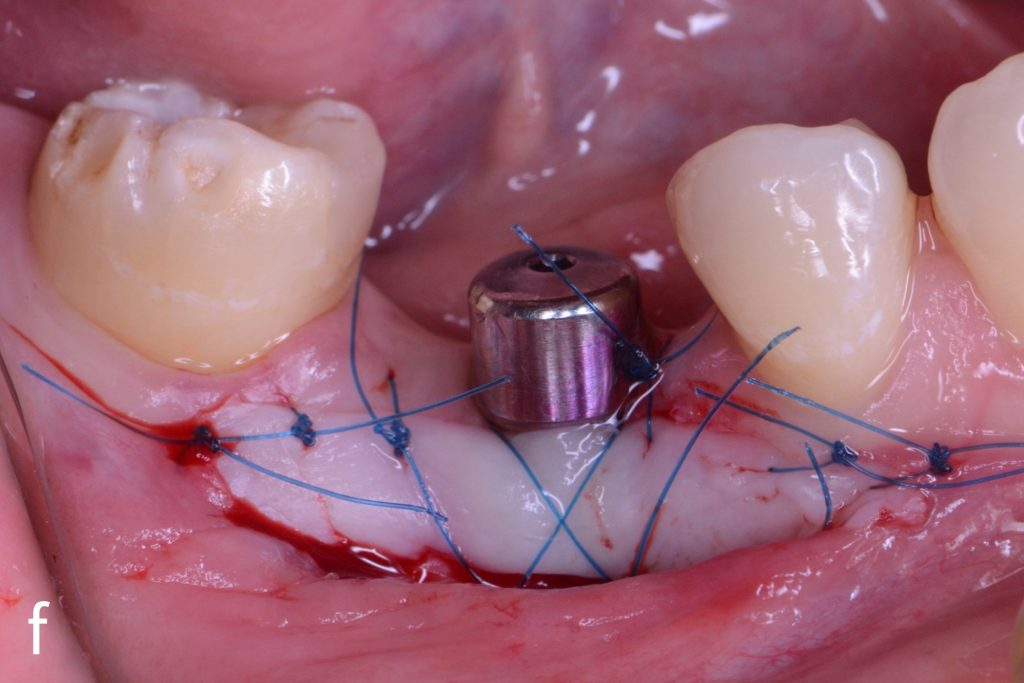
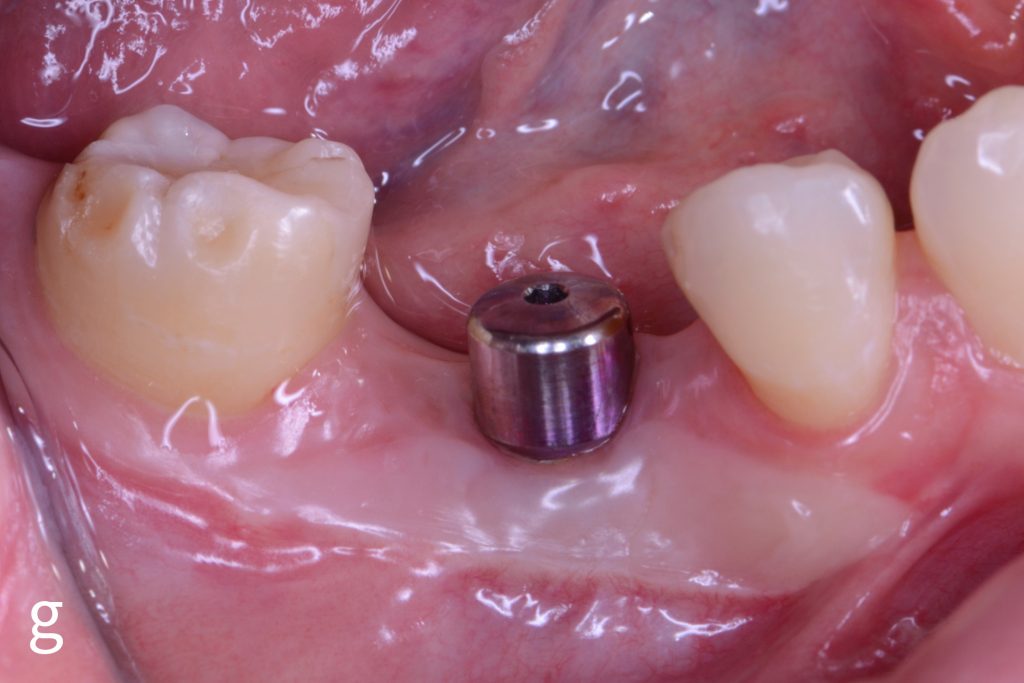
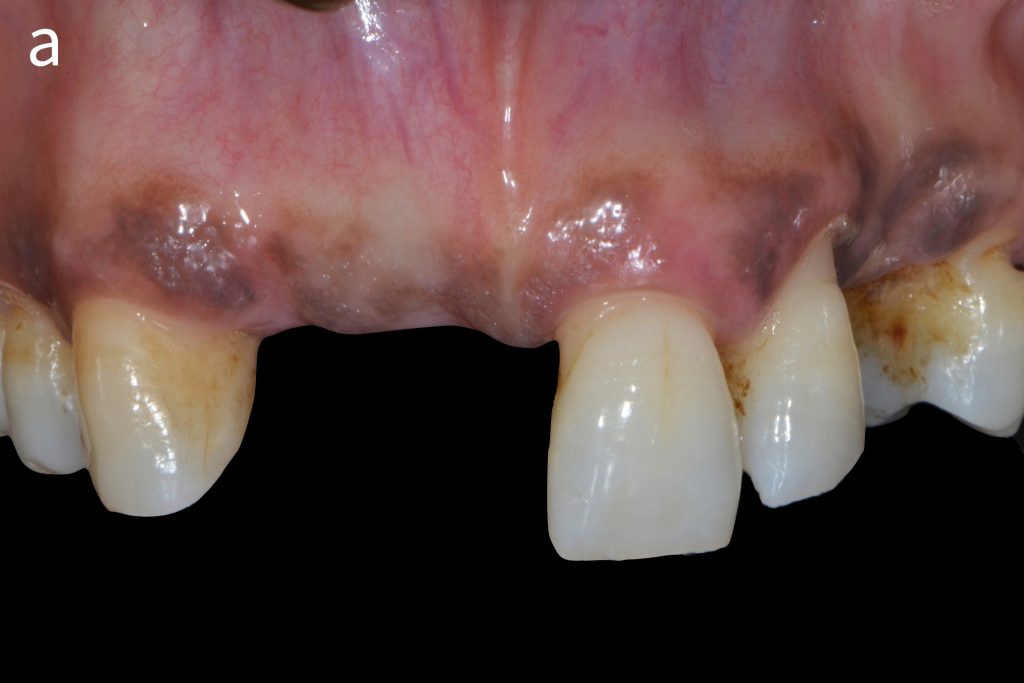
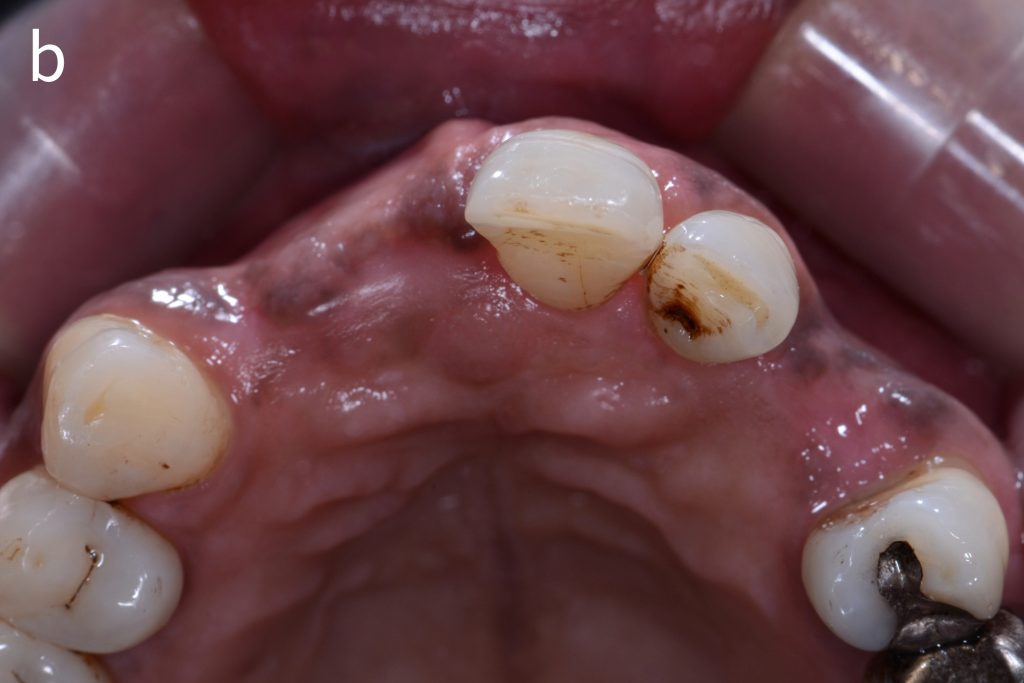
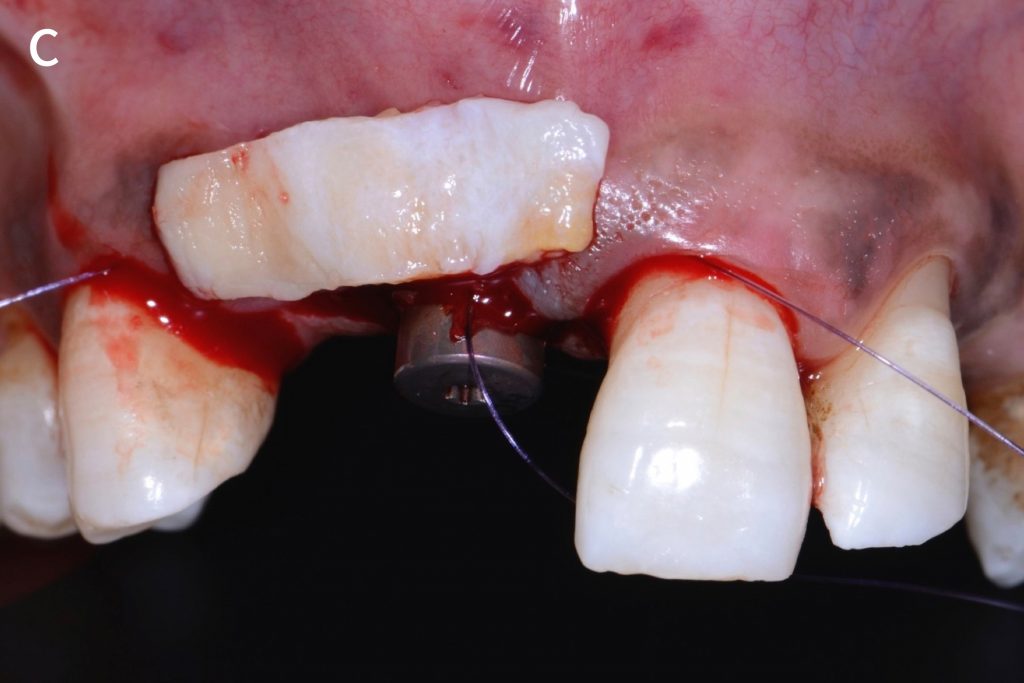
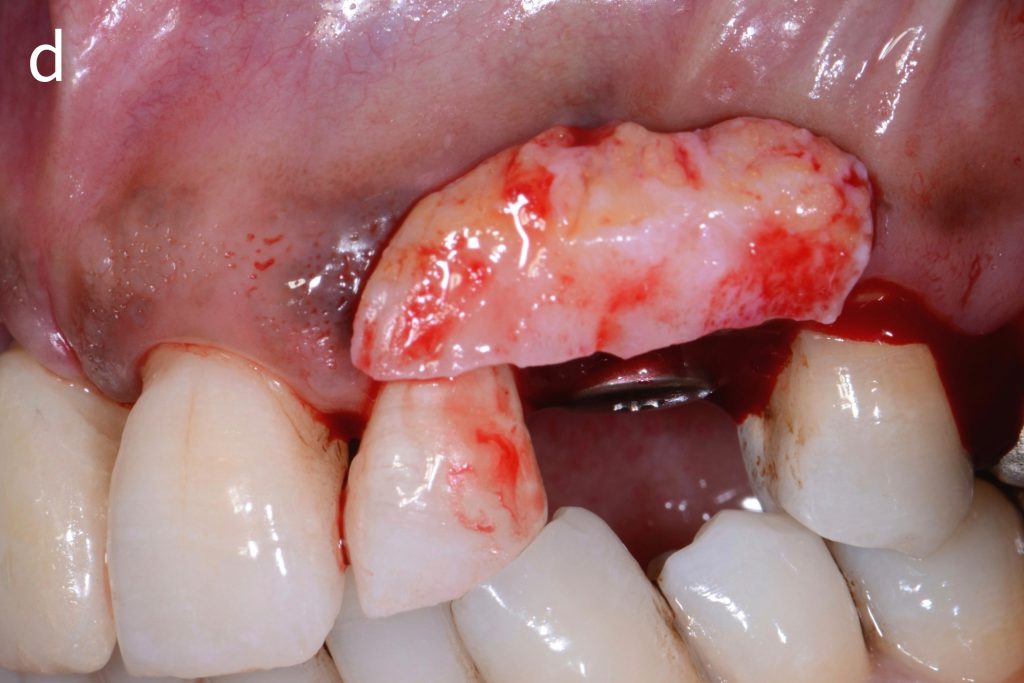
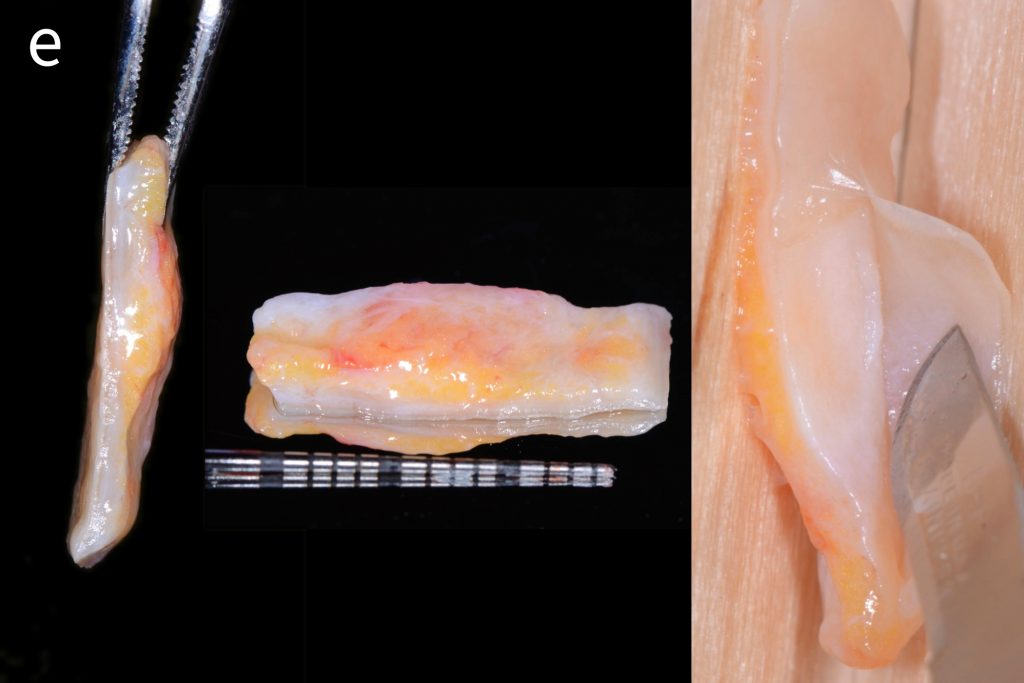
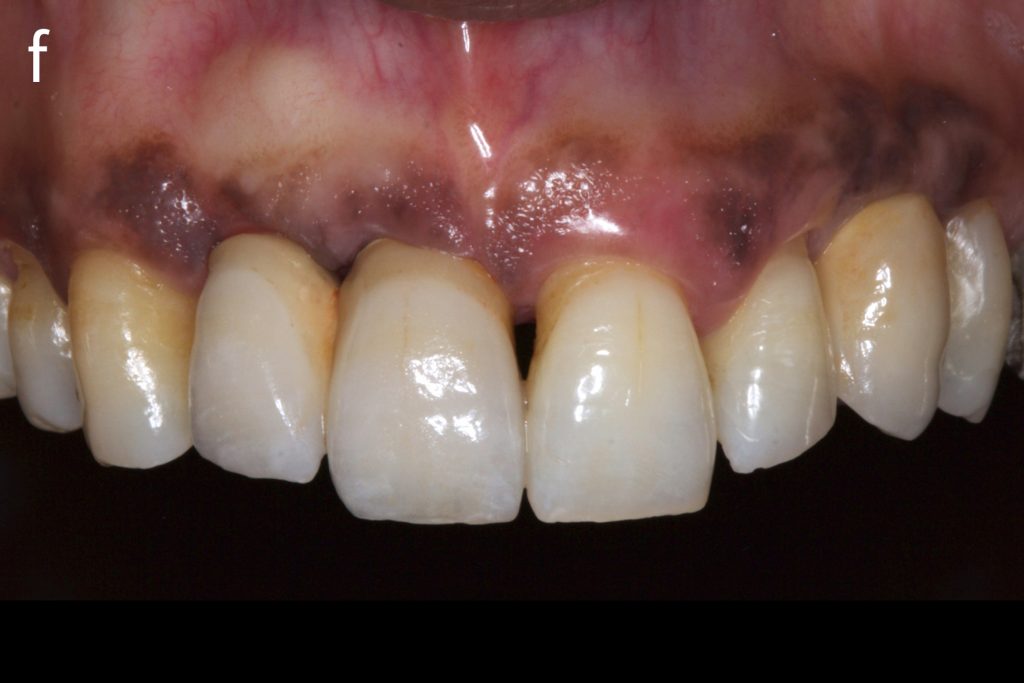
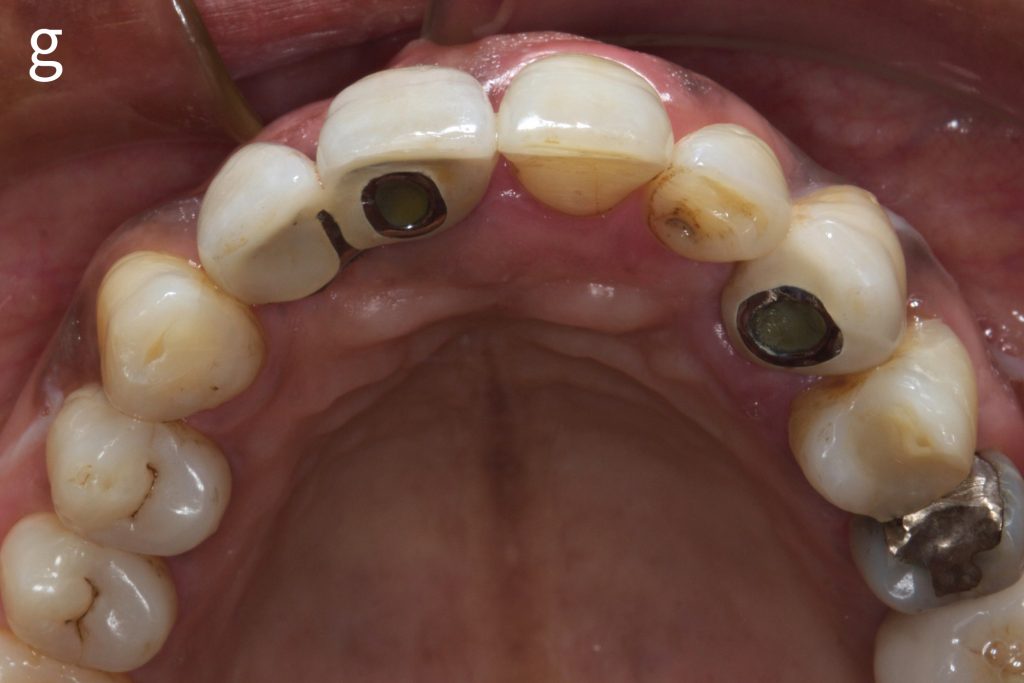
Figs 2a – g: Connective tissue graft procedure around dental implants
In that above clinical case, there are two implants placed at the patient’s maxillary right central incisor and maxillary left canine to restore the edentulous ridge. The concavity over the buccal side of the dental implants indicate the deficiency of soft tissue after implant osteointegration (Figs 2a – b). To achieve a better esthetic outcome of the final prosthesis, subepithelial connective tissue grafting was performed simultaneously with the implant second-stage surgery to increase peri-implant mucosal thickness. The tunnel technique was used to prepare the recipient site in the area of the dental implant and subepithelial connective tissue was harvested from the patient’s hard palate. The graft was inserted and a suture applied at the recipient site (Figs 2c – e). Although the patient was diagnosed with severe chronic periodontitis, where it is challenging to achieve esthetic result, the final result showed a harmonious soft tissue outline around the final prosthesis after soft tissue management (Figs 2f – g).
Summary
There are many soft tissue grafting techniques that can be used for gingival augmentation and soft tissue grafting procedures around dental implants. Surgeons can choose a suitable technique for improving the pink esthetic around dental implants not only by increasing the “quantity” but also by enhancing the “quality” to achieve a long-term stable esthetic outcome.