Background and objective
After tooth extraction, a remodeling process takes place in the alveolar socket, leading to volumetric changes that reduce bone availability for further dental implant placement. Why do dimensional alterations happen after tooth removal? Briefly, the periodontal ligament is lost during the alveolar healing process, which consequently affects the maintenance of the bundle bone (Araújo & Lindhe, 2005). Bundle bone is an anatomical structure in which periodontal ligament fibers insert. After tooth extraction, the periodontal ligament is lost and so is the bundle bone. As the bundle bone deteriorates, the alveolar bone that surrounds the tooth is resorbed, especially in individuals with a thin periodontal phenotype (Chappuis et al., 2013). This phenomenon is more intense in the first few months after tooth extraction, but it is continuous, and will progressively lead to a reduction in alveolar ridge volume, sometimes precluding an adequate implant-supported rehabilitation (Mardas et al., 2015). Therefore, some clinical strategies should be employed to compensate the post-extraction dimensional alterations of the alveolar ridge.
According to our philosophy, every time a patient presents a hopeless tooth, it is important to consider post-extraction immediate or early implant placement. However, alveolar ridge preservation has its indications, particularly when implant placement is to be delayed (Avila-Ortiz et al., 2020). Even though alveolar ridge preservation involves other important steps such as minimally invasive tooth extraction and filling the alveolar socket with a slowly resorbing biomaterial, our focus is on another important step: alveolar socket sealing. Hence, the objective of this report is to discuss three different approaches for sealing alveolar sockets in the context of alveolar ridge preservation.
Alveolar ridge preservation
Alveolar ridge preservation refers to a method that aims to minimize the loss of ridge dimensions after tooth extraction and avoid more demanding surgical procedures in the future. To accomplish this objective, placing a bone graft material into the post-extraction socket has been proposed in combination with socket sealing using autogenous soft tissue plugs or barrier membranes. Alternatively, non-autogenous matrices have been used to replace soft tissue grafts, thus reducing morbidity to patients since they exclude access to donor sites for tissue harvesting. In the case of a hopeless tooth with the decision to carry out an alveolar ridge preservation procedure, delayed implant placement can be performed six months after tooth removal (Avila-Ortiz et al., 2019).
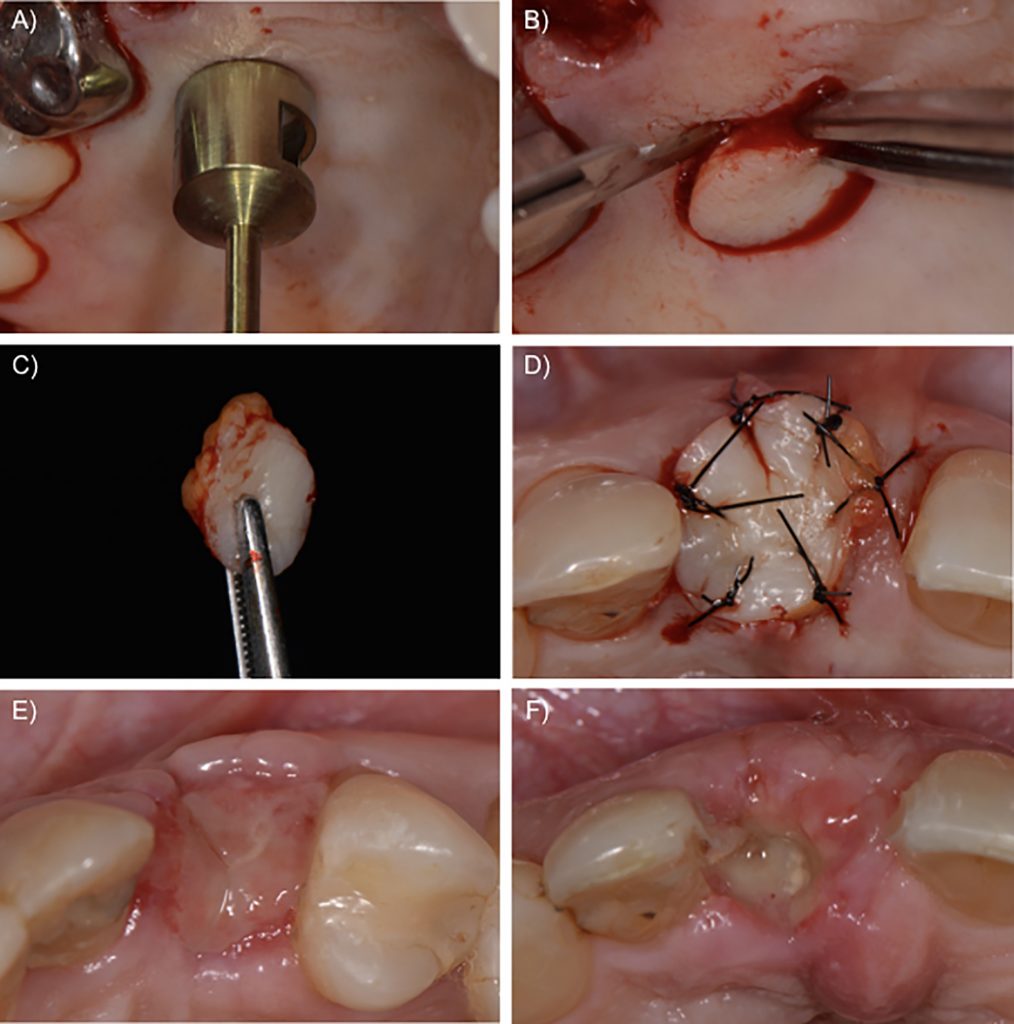
Considering the clinical sequence for alveolar ridge preservation, our protocol starts with a flapless minimally invasive tooth extraction, carried out as gently as possible to minimize the inflammatory remodeling process due to surgical trauma. After cutting the gingival fibers with a scalpel, a periotome is used to luxate the tooth by cutting the periodontal ligament fibers, especially in the interproximal areas. Next, a vertical root extraction system can be employed to remove the fractured tooth, thus avoiding the use of elevators or forceps. First, a root canal is prepared, and then a traction system is attached to the tooth and the root is pulled out without putting pressure on the surrounding tissues, causing minimum trauma to neighboring structures. It is important to emphasize that minimally invasive extraction is critical in order to maintain the buccal bone wall and the interproximal papilla, which is crucial to the success of alveolar ridge preservation (Fig. 1).
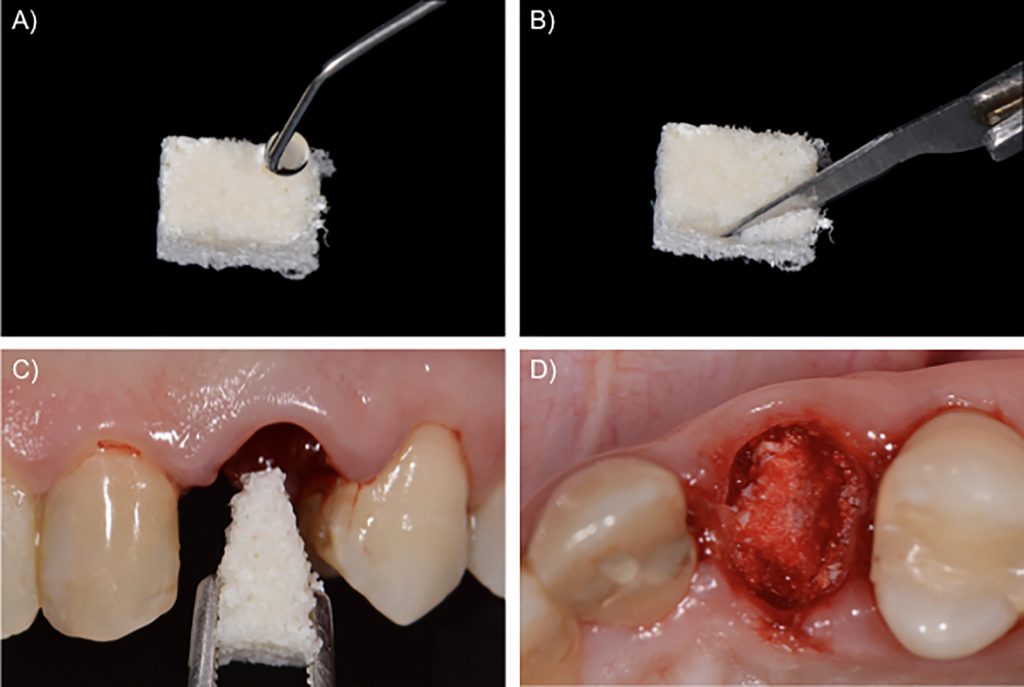
The next step is to fill the fresh alveolar socket with a slowly resorbing biomaterial. We used a xenograft based on deproteinized bovine bone mineral with 10% collagen to improve its handling (Llanos et al., 2019). First, the biomaterial is moistened with saline solution and then sliced up with a sharp blade. Finally, the prepared biomaterial is introduced in the alveolar socket up to the apical portion. The remaining biomaterial is placed in the gaps inside the socket up to the level of the alveolar bone crest (Fig. 2). Once the socket is filled with the biomaterial, the socket opening should be closed – there is controversy on the most appropriate method (Avila-Ortiz et al., 2019). There are three possible approaches that we illustrate in this report: the use of a free autogenous soft tissue graft from the palate, an acellular collagen matrix, or a collagen membrane.
Case 1 – Free autogenous graft from the palate
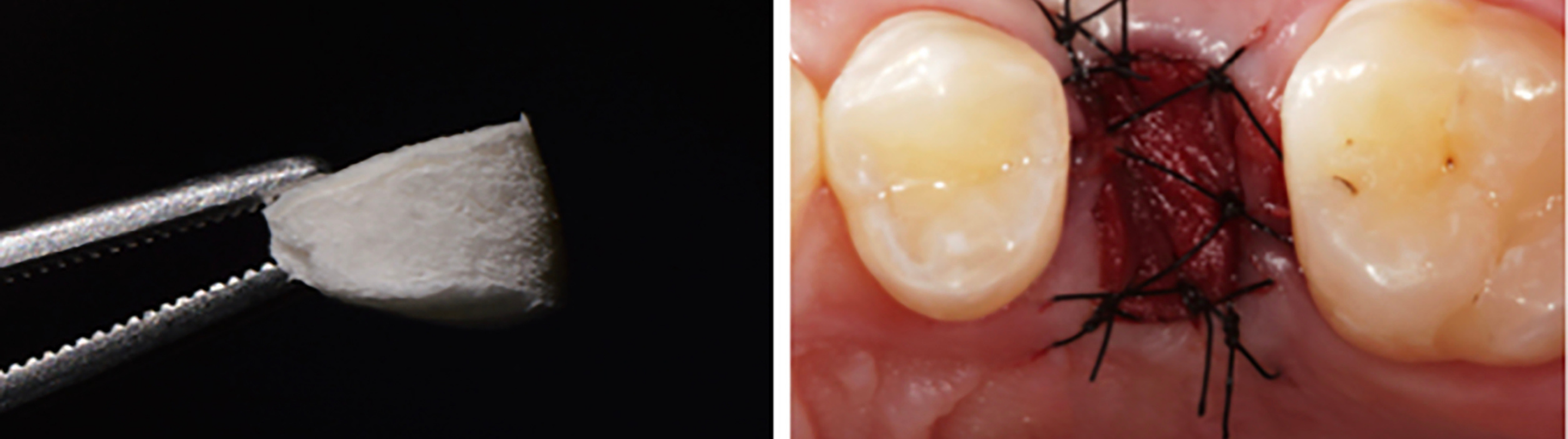
The soft tissue from the palate is currently the reference and probably the most frequently used technique for socket sealing in alveolar ridge preservation. The procedure normally involves an incision in the palatal area, which may be performed with a punch blade or a scalpel corresponding to the oval outline of the socket. The tissue can be held with tweezers and the incision can be completed at the bottom of the tissue up to its removal. After the de-epithelialization of the marginal tissue of the receptor site with a scalpel or diamond bur, the epithelialized soft tissue graft is placed on the socket, over the bone substitute material, and sutured to the borders of the alveolar cavity in the receptor area. The objective of these sutures is to stabilize the autogenous graft during the first days or weeks after the surgical procedure. The nutrition of these grafts, however, is deficient, and two situations are possible in the postoperative follow-up: incorporation of the soft tissue graft, or necrosis of the grafted tissue. In most cases, granulation tissue forms underneath the soft tissue graft, which maintains the integrity of the biomaterial inside the alveolar socket. Nevertheless, the soft tissue graft may be lost too soon, exposing the bone substitute and impairing the bone formation inside the socket. Although not always present, one disadvantage of the autogenous graft is scar formation between graft and surrounding tissue, which may affect aesthetic outcomes (Fig. 3).
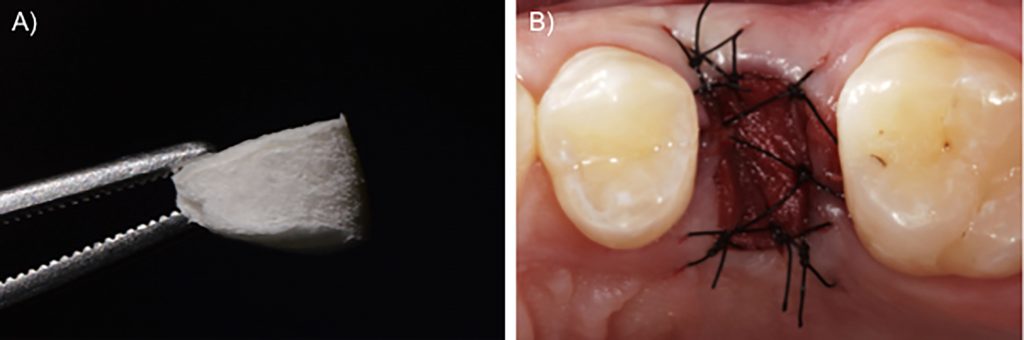
Case 2 – Collagen matrix
Some heterogenous and xenogenous matrices were developed as alternatives for free autogenous grafts. More recently, a collagen-based matrix was introduced in the market specifically for use sealing the alveolar socket. This porcine-derived material is constituted of two layers: a lower, spongy layer that is thicker, approximately 5 mm when dry; and a compact, upper layer that is thinner, approximately 1 mm thick, with a dense arrangement of collagen fibers. This lower layer with open spaces between the struts of the collagen network is responsible for blood-clot stabilization, which may facilitate cell ingrowth and further tissue formation. On the other hand, in the upper part, the collagen fibers are tightly organized, which provides enough resistance when suturing the material to the surrounding tissues and delays the degradation process, which is good, since the material needs to remain in situ at least until healing tissue has formed (Sanz et al., 2009). To stabilize the collagen matrix on the surgical site, single interrupted sutures can be performed from the borders of the material to the soft tissues around the alveolar socket. Since this material loses its thickness when soaked, we recommend suturing the material dry, the collagen matrix will gradually become wet during suturing (Fig. 4).
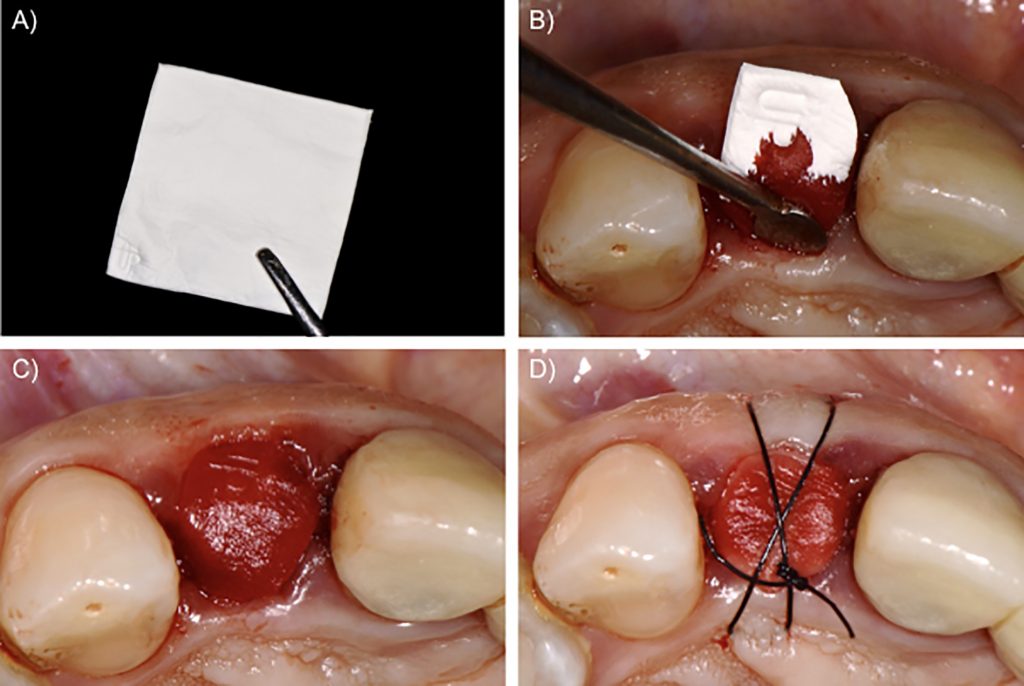
Case 3 – Collagen membrane
There is solid evidence of good results with collagen membranes for guided bone regeneration and bone augmentation procedures (Naenni et al., 2019). However, their clinical performance for socket sealing is still controversial. Clinicians usually employ collagen membranes as a socket sealing material when defects of the alveolar bone walls are present. In these situations, collagen membranes are cut in the shape of the alveolar defect and placed over the bone substitute, with the borders of the membrane carefully adjusted under the marginal gingiva (Fig. 5). Even though some studies demonstrate the potential for the use of collagen membranes exposed to the oral cavity (Choi et al., 2017; Lim et al., 2019), this use does not have the same evidence of success as for guided bone regeneration. Exposure to the oral cavity may accelerate the degradation of the membrane, leading to early exposition of the bone substitutes inside the alveolar socket (Garcia et al., 2018).
Nevertheless, we can benefit from the use of collagen membranes in alveolar ridge preservation by employing the membrane as a secondary sealing material under a soft tissue graft or acellular matrix. In a clinical study, alveolar sockets were volumetrically preserved with xenogenous grafts and sealed with soft tissue pedicles from the palate, with or without collagen membranes underneath (Perelman-Karmon et al., 2012). The membrane-protected sockets showed increased bone formation as compared to unprotected sockets, suggesting that a “double sealing” strategy could be potentially interesting for alveolar ridge preservation procedures.
Concluding remarks
The socket sealing strategies proposed in this article, with autogenous graft, collagen matrix or collagen membrane, are very much alike in terms of their potential for assisting alveolar ridge preservation. We speculate that the socket sealing material has the single function of remaining in situ long enough for the formation of a granulation tissue beneath it, which would cover the graft material inside the socket and give way to keratinized mucosa after maturation. However, this hypothesis remains to be clarified in future research. Xenogenous biomaterials for socket sealing do not increase postoperative morbidity since they do not require surgical access to a donor site. However, clinicians should consider that biomaterials add costs to the treatment and the decision to replace autogenous grafting techniques must be taken on a case-by-case basis, according to the patients’ needs.