Introduction
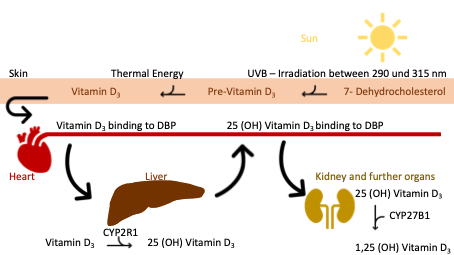
Vitamin D is a steroid hormone that can be synthesized in the skin with sufficient sunlight (290-315 nm) and converted into an active form in the liver and kidneys (Lehmann, 2005). Adequate sunlight for endogenous vitamin D synthesis is only available from April to September at a north latitude of 40 degrees. This corresponds to the geographical areas north of Madrid (O’Connor, 2011), so there is a risk that many people in Europe, North Asia, and North America will be insufficiently supplied with vitamin D from sunlight alone.
The human body cannot synthesize sufficient vitamin D all year round. Vitamin D is available in various forms through diet. Ergocalciferol (vitamin D2) is found in plants, while cholecalciferol (vitamin D3) is found in animal foods (Ross et al., 2011; Rep Health Soc Subj., 1998). Depending on the type of hydroxylation, a distinction is made between active and inactive forms of vitamin D. Foods such as fatty fish, liver (beef), eggs, milk, cheese, soy, and mushrooms contain a lot of vitamin D (Holick & Chen, 2008; US FDC, 2021). However, the supply of vitamin D from food tends to play a subordinate role, and vitamin D plays a vital role in mineral homeostasis, as it stimulates the intestinal absorption of calcium and phosphate (Halfon et al, 2015). It regulates bone metabolism and bone mineralization by inhibiting osteoclast genesis and bone resorption while activating osteoblasts (Holick, 2007; Atkins et a. 2020). Laboratory measurements determine the 25(OH)-vitamin D status, as it is a metabolite with a high concentration and a relatively long half-life of several weeks to months. The active version is 1.25(OH)-vitamin D. Since a laboratory chemical measurement can only detect the level of 25(OH)-vitamin D in the bloodstream, it is not a completely accurate method for measuring the vitamin D available in the whole body. However, it is a meaningful measurement of the available vitamin in the bloodstream and an established marker for vitamin D levels (Rep Health Soc Subj., 1998; Makris et al., 2020).
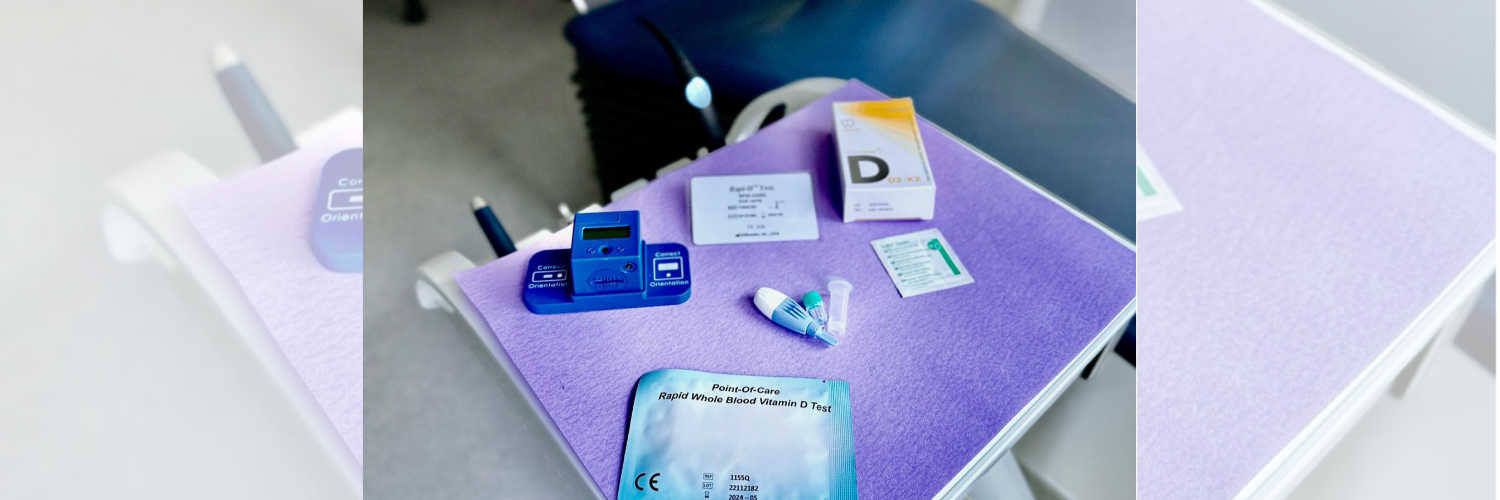
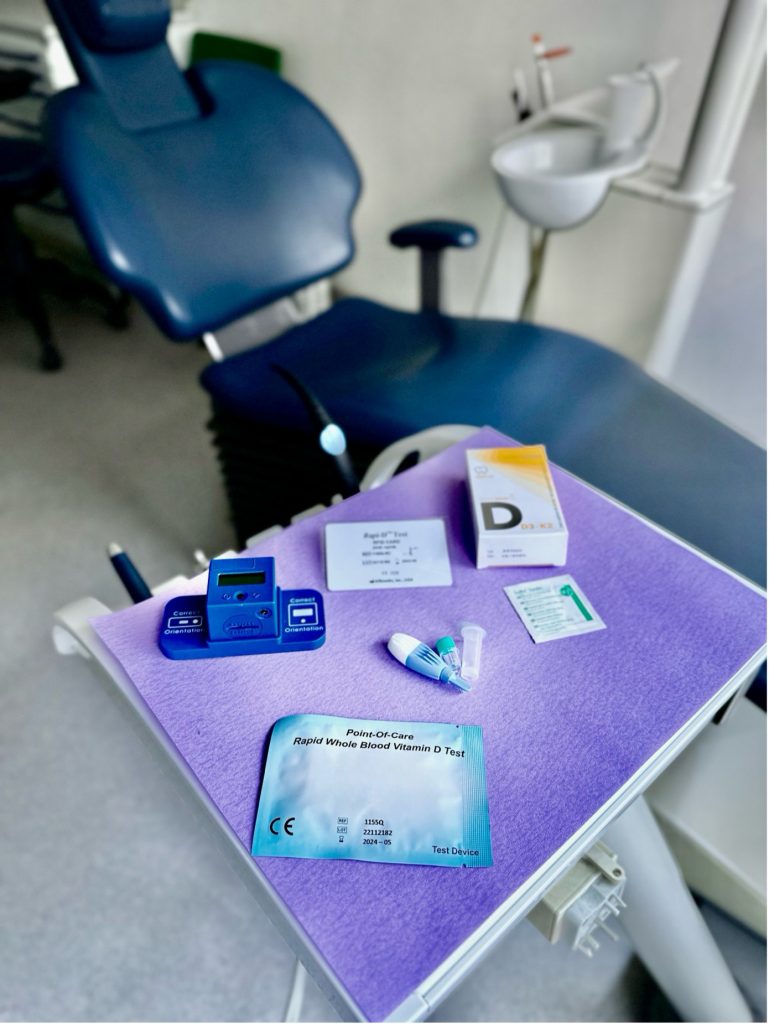
Laboratory measurement has long been the only option for determining patients’ vitamin D levels. Recently, a minimally invasive, chairside rapid vitamin D test was developed.



Incidence
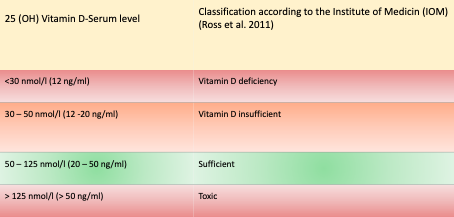
Serum 25(OH)-vitamin D (1ng/ml corresponds to 2.5nmol/l) is the laboratory chemical parameter for the medical determination of vitamin D status. A blood 25(OH) vitamin D serum level of less than 30nmol/l (12ng/ml) is considered “deficient” because it is associated with the development of rickets (Holick, 2007). Apart from this, little agreement exists on an “optimal” blood vitamin D level. There is no standard definition or agreement (Cashman and Kiely, 2014). Approximately 20-100% of the European, US, and Canadian population over the age of 65 are vitamin D deficient, as defined by the American Institute of Medicine (IOM) (Holick, 2007).
The population between 1 and 65 years has a comparable risk of vitamin D deficiency or insufficiency. There is also a consensus that older population groups, especially post-menopausal women worldwide, often have inadequate serum vitamin D levels (Hilger et al., 2014). In addition, 80% of young people from 9 Central European countries had suboptimal 25(OH)-vitamin D levels. Thus, 27% had a deficiency of 25(OH)-vitamin D (27.5-49.9nmol/l) and 15% even had a severe deficiency (<27.5nmol/l) (Singh et al., 2006). Therefore, the probability of suffering from a vitamin D deficiency or a suboptimal vitamin D supply is high, regardless of age. In addition, people exposed to little or no sunlight (Cashman and Kiely, 2014) or who are heavily pigmented (Holick, 2004) belong to the risk group for vitamin D deficiency. Added to this are people with a restricted or monotonous diet, obesity, or a high body mass index (BMI) (Souberbielle et al., 2006). This may also include people with a diet low in vitamin D, such as vegans and vegetarians (Crowe et al., 2011). People who, for social or health reasons, spend little or no time outdoors and are not exposed to sunlight are particularly at risk of vitamin D deficiency.
Vitamin D deficiency is associated with a variety of diseases. These include periodontitis (Laky et al., 2017; Antonolglou et al. 2015), premature tooth loss (Zhan et al., 2014), catabolic metabolic conditions, osteoporotic fractures (Holick, 2007), and impaired fracture healing (Brinker et al.,2007; Alkalay et al., 1989). However, vitamin D supplementation did not significantly improve the occurrence or healing of fractures (Gatt et al., 2023; Jackson et al., 2006). It should be noted that indiscriminate vitamin D supplementation can also have harmful effects and is therefore not recommended (Jungert et al., 2020). Substitution should only be carried out after prior determination of the 25(OH)-vitamin D value adjusted to this.
Causes
In northern Europe, vitamin D deficiency is generally due to insufficient synthesis in the body in connection with inadequate exposure to sunlight or a decrease in the skin’s ability to synthesize vitamin D with age.
Consequences
A reduced concentration of vitamin D leads to a decrease in the effectiveness of intestinal absorption of calcium and phosphate from food, which reactively increases the level of circulating PTH (parathyroid hormone) (Lips et al., 2006). Secondary hyperparathyroidism increases the serum calcium level to a suboptimal range (serum concentration of total calcium >2.65 mmol/l). The resulting demineralization of the bone causes calcium to be mobilized from the skeleton due to increased osteoclast activity. This leads to increased excretion of phosphate by the kidneys. Another consequence of secondary hyperparathyroidism is phosphaturia. This leads to a compensation of the serum phosphate level into the normal range or below. This results in an imbalance in the phosphate-calcium product, which causes a mineralization deficit in the skeleton (Grober et al., 2013). The consequence is reduced bone density, leading to osteopenia and osteoporosis.
Clinical significance
Periodontitis
Inadequate vitamin D levels appear to be involved in the progression of periodontal disease (Laky et al., 2017) and periodontitis (Antonoglou et al., 2015). Study results indicate that periodontal disease significantly correlates with low 25(OH) vitamin D levels. Whether the effect is only on the tissue, the immune cells, the microorganisms (Grenier et al., 2016), or several elements still needs to be clarified. In experimental studies, vitamin D had inhibitory effects at low concentrations on microorganisms such as P. gingivalis, A. actinomycetemcomitans, F. nucleatum, and S. moorei, which are involved in developing periodontitis. However, very high doses of vitamin D showed bactericidal effects in in-vitro experiments (Grenier et al., 2016). Vitamin D inhibited the proliferation of P. gingivalis by significantly lowering the expression of virulence factor genes, which are essential in bacterial colonization, deactivation of host defense mechanisms, tissue destruction, and food procurement. After vitamin D binds to its receptor, it exerts its effect on the myeloid cells of the immune system, among others. It has an inhibitory effect on T lymphocytes through reduced proliferation and lower production of interleukin-2 (IL-2) and interferon-γ (IFN-γ) (Cippitelli and Santoni, 1998).
A lack of vitamin D can lead to deficits in all these positive developments. It is also known that in the presence of vitamin D in the periodontal space, fewer inflammatory mediators and cytokines, such as IL-8 and CC chemokine ligand-2 (CCL2), are present (Andrukhov et al., 2014). However, the concentration of vitamin D at which this effect was measured has not been clearly described. When vitamin D was added, human periodontal fibroblasts also observed increased expression of osteopontin, osteocalcin mRNA, and increased alkaline phosphatase activity. As a result, inflammation and tissue destruction were reduced (Nebel et al., 2015). Studies have shown that substituting calcium and vitamin D positively affected supportive periodontal therapy (SPT) (Garcia et al., 2011). In addition, low vitamin D levels are associated with a greater risk of tooth loss overall (Zhan et al., 2014). The extent to which these effects are solely due to vitamin D deficiency and not to other environmental factors remains unclear and needs to be investigated in further studies.
Osseointegration of implants
One animal study demonstrated that rats with sufficient vitamin D levels when treated with implants showed higher values regarding bone-implant contact, implant stability, and osseointegration than those with a vitamin D deficiency (Kelly et al., 2009). Further animal studies have shown that osseointegration of implants after vitamin D supplementation was better in animals with pre-existing conditions such as chronic osteoporosis, renal insufficiency, or diabetes mellitus than in animals with the same pre-existing conditions without vitamin D supplementation (Wu et al., 2020; Zhou et al., 2012; Liu et al., 2014). In healthy animals with an adequate vitamin D supply, supplementation with vitamin D, magnesium, and calcium was not associated with improved osseointegration compared to healthy animals without supplementation (Pimentel et al., 2016). Whether these results from animal experiments can be directly transferred to humans remains unclear. Individual case reports have linked early implant failures to inadequate vitamin D serum levels (Fretwurst et al., 2016; Bryce & MacBeth, 2014). In addition, a retrospective study of over 880 patients showed that low vitamin D levels were associated with an increased risk of early implant failures. However, no significant correlation was found (Guido Mangano et al., 2018). Therefore, it cannot be assumed at this time that vitamin D supplementation has a positive effect on implant survival. Further studies are needed to provide clarity in this area.
Caries prevention
In the 1920s and 1930s, many experiments were carried out that linked vitamin D supplementation with a reduced incidence of caries. Little attention was paid to this topic until 2013. A systematic review by Hujoel (2013), which included several studies from the early 20th century, suggested that vitamin D supplementation was associated with a 47% reduction in caries risk. It was also shown that the source of vitamin D (substitution of vitamin D2, vitamin D3, or endogenous vitamin D3 after UV light exposure) had no significant influence on reducing the risk of caries (Hujoel, 2013). In 2 studies on schoolchildren, it was shown that children who were taught in a classroom with full-spectrum light had a significantly lower caries incidence on 6-year molars and a substantially lower increase in the DMFS score (“decay-missing-filled surfaces index”) than children who were illuminated with conventional light during the same period (Mayron et al., 1975; Hargreaves & Thompson, 1989). The caries-protective effect of ultraviolet light shown in clinical and preclinical studies may be due to increased endogenous synthesis of vitamin D. However, the study design does not allow the conclusion of a causal relationship attributing the cause of caries protection to vitamin D alone. Research results from the last decade underline the earlier discoveries. It was found that children with higher serum 25(OH) vitamin D levels had a significantly lower rate of dental treatment under general anesthesia than children with lower serum levels (Dudding et al., 2015). In addition, a significantly lower caries incidence was found in children with a 25(OH) vitamin D serum level of over 50 nmol/l (Schroth et al., 2016). Carvalho Silva et al. (2021) obtained similar results, but the children had a significantly higher risk of developing caries on permanent teeth at a serum level of less than 75 nmol/l. In addition to the previously presented caries-protective effects of vitamin D, a significantly reduced rate of molar incisor hypomineralization (MIH) on permanent teeth was also found in children with higher 25 (OH) vitamin D serum levels (Kuhnisch et al., 2015).
The present literature on vitamin D deficiency and oral health suggests that vitamin D levels may influence oral health. Further clinical research is required to support this hypothesis.