The burden of osteoporosis
Osteoporosis is a common skeletal disease characterized by reduced bone mineral density (BMD) and changes in the microarchitectural structure of bones which predispose patients to an increased risk of fractures. It is often referred to as a “silent disease” as many people become aware they have osteoporosis only after a fracture occurs.
The gold standard for the diagnosis of osteoporosis is related to the measurement of BMD through dual energy X-ray absorptiometry (DXA) at the hip and lumbar spine. In particular, BMD is defined in relation to a T-score that describes the number of standard deviations by which the BMD recorded differs from the mean value expected in young healthy individuals. The World Health Organization (WHO) defines osteoporosis as a T-score 2.5 or more below the young female adult mean (Kanis et al., 2008), while osteopenia (i.e., reduced BMD) corresponds to a T-score between -1 and -2.5 (Table 1).
WHO Operational Definition of Osteoporosis based on BMD measurement
| Classification | T score |
| Normal | ≥-1 |
| Osteopenia | -1<T<-2.5 |
| Osteoporosis | ≤-2.5 |
| Severe or established oteoporosis | ≤-2.5 + one or more fractures |
In 2010 it was estimated that osteoporosis had a prevalence of 27.6 million in Europe (22 million women and 5.6 million men), with 3.5 million new fragility fractures recorded. These data are expected to significantly increase in the future (+23% by 2025), owing to population growth and ageing (Hernlund et al., 2013). The prevalence of osteoporosis is the highest in Caucasians, as approximately one in two Caucasian women and one in five men are expected to experience an osteoporosis-related fracture in their lifetime. Women after menopause are particularly at risk of developing osteoporosis, due to the negative impact that oestrogen withdrawal has on bone metabolism.
Considering the increase in life expectancy, the number of osteoporotic patients requiring dental care, including implant rehabilitations, is expected to significantly rise in the coming years and therefore it is important for dentists to be aware of any possible detrimental effect of osteoporosis (and its medications) on the jawbones in order to reduce the incidence of complications and improve the success of dental treatments in this group of patients.
Does osteoporosis affect the jawbones?
It is plausible to think that osteoporosis-induced systemic bone loss may also include bone loss at the jaws, as part of the skeleton. As a matter of fact, several studies found a positive correlation between BMD measured at different skeletal sites and jawbone density (Erdogan et al., 2009, Drozdzowska et al., 2002, Jonasson et al., 2001, Makker et al., 2012, Takaishi et al., 2005, Vishwanath et al., 2011, Horner et al., 1996, Esfahanizadeh et al., 2013), with increased alveolar bone resorption in osteoporotic versus non-osteoporotic edentulous patients (Hirai et al., 1993, Singhal et al., 2012). However, other studies did not confirm these findings, thus making the available evidence uncertain.
One of the biggest limitations standing in the way of making more robust conclusions is the fact that we lack a standardized and accurate technique to measure jawbone density, since a DXA software for jawbones does not exist (Calciolari et al., 2015b). Currently, quantitative computed tomography remains the most reliable pre-surgical assessment of jaw BMD, and one study suggested Hounsfield unit (HU) cut-offs for identifying osteoporotic patients in the dental practice (460 HU for spine T-score) (Chai et al., 2014).
Despite their controversial results, in the past 30 years many studies have claimed a role for the dentist in the early diagnosis of osteoporosis by measuring specific quantitative/qualitative indices on dental panoramic radiographs (OPGs). The idea is that, since OPGs are frequent exams available in dental practices and often performed during check-ups or in association with dental treatments, the dentist could use them to intercept patients at risk of osteoporosis and refer them to a specialist early on, before any fractures take place (Calciolari et al., 2015a). At the moment, the identification of patients at risk of osteoporosis through OPGs is at an experimental/research level, but the results seem promising and it is possible that in the near future and with the help of automatic and computer-based systems this might become a solid reality.
Can we place dental implants safely in osteoporotic patients?
It is biologically plausible that the alterations in bone metabolism associated with osteoporosis (namely increased bone resorption and reduced bone density) can also impair bone healing around dental implants and negatively affect their osseointegration.
While studies in animals overall suggest that osteoporosis is associated with reduced bone-to-implant contact and reduced mechanical properties (Dereka et al., 2018), the results of prospective controlled studies indicate similar survival and success rates for implants placed in healthy and in osteoporotic patients (Bornstein et al., 2009a, de Medeiros et al., 2018). As such, osteoporosis should not currently be considered as a contraindication for implant placement. Nevertheless, clinicians might want to follow some recommendations based more on expert opinion rather than solid scientific evidence, with the aim to improve the predictability of implant-related outcomes when dealing with osteoporotic patients.
In particular, clinicians should carefully assess and try to check for concomitant risk factors that can affect bone metabolism and bone density (such as deficiencies of vitamin D and calcium, smoking, alcohol abuse) as well as for the presence of systemic diseases, such as diabetes mellitus, with a recognized impact on bone tissue. It is also suggested to consider osteoporotic bone as equivalent to type IV in the Lekholm and Zarb classification, thus porous and on average of poor quality. Therefore, when preparing the implant site, clinicians might take into consideration longer healing periods before sitting the prosthesis, paying particularly attention on the insertion torque analysis, especially if immediate loading is considered. Resonance frequency analysis might also be a useful tool to assess implant stability before loading, although limited evidence is available on the correlation between skeletal osteoporosis and dental implant stability (Merheb et al., 2016).
A rapidly growing research field is now related to the use of implant surfaces that can be bioactivated, drug loaded or chemically modified to enhance osseointegration and bone formation, especially in compromised healing conditions, such as osteoporosis.
For instance, it is well documented that hydrophilic micro-rough titanium implants can accelerate the initial stages of osseointegration by promoting interactions with osteogenic cells, biological fluids and tissues and by promoting osteoblast differentiation (Bornstein et al., 2008, Buser et al., 2004, Donos et al., 2011). Clinical trials have acknowledged the successful use of these modified implant surfaces for immediate and early loading (up to 3 weeks after implant placement) and in challenging anatomical situations (such as type IV bone) (Morton et al., 2010, Bornstein et al., 2009b, Zollner et al., 2008, Roccuzzo and Wilson, 2009), so it is likely that these modified surfaces might also be indicated in osteoporotic patients to ensure more predictable outcomes, although no clinical studies have been published in this respect so far.
What is the effect of osteoporosis medications on the success and survival of dental implants?
There are two categories of osteoporosis medication: antiresorptive (anticatabolic) and anabolic agents (Table 2). Antiresorptive medications act by reducing the number and activity of osteoclasts and therefore slowing down bone turnover, while anabolic medications increase the frequency of bone remodelling and promote bone formation.
FDA-approved medications for osteoporosis
| Antiresorptive | Alendronate (Fosamax, Fosamax Plus D) Risedronate (Actonel, Actonel with calcium) Ibandronate (Boniva) Zoledronic acid (Reclast) Oestrogen therapy/hormone therapy Raloxifene (Evista) Denosumab (Prolia) Bazedoxifene and estrogen (Duavee) Calcitonin salmon (Fortical, Miacalcin) |
| Anabolic | Teriparatide (Forteo) Abaloparatide (Tymlos) Romosuzumab (Evenity) |
Bisphosphonates (BPs), and in particular alendronate, are still the most commonly prescribed antiresorptive medications for osteoporosis and represent the gold standard for fracture prevention. They can be administered either orally (more frequently) or intravenously and they are stored in bones for decades due to their strong affinity for hydroxyapatite.
Denosumab is a human monoclonal antibody against the receptor activator of the nuclear factor kappa-B ligand (RANKL) protein and therefore has an inhibitory effect on osteoclast maturation, activation and survival. It is administered as a single subcutaneous injection once every 6 months. Remarkably, denosumab presents with an anti-fracture activity similar to zoledronic acid, but it does not have residual effects beyond 6 months.
Since antiresorptive medications inhibit the formation and activation of osteoclasts and induce their apoptosis, thus reducing bone turnover, they may potentially reduce the regenerative capacity of bone around dental implants, thus negatively affecting the osseointegration process. At the same time, the slower osseous remodelling allows more time for secondary mineralization, which leads to an increase in bone density and stiffness, together with an increase in bone microdamage.
Although the available clinical evidence comes mainly from retrospective and case series studies, it seems that systemic BPs do not increase implant failure or peri-implant marginal bone loss, as also reported at the 6th ITI Consensus Conference (Chappuis et al., 2018). The number of dental implants that must be exposed to BPs in order to cause a single implant failure which otherwise would not have occurred (“number needed to harm”) was reported to be 509 dental implants (Ata-Ali et al., 2014).There is almost no information available on the possible effect on implant therapy of denosumab, nor on the success or safety of bone grafting procedures in patients taking antiresorptive medications.
Although antiresorptive medications do not seem to significantly reduce implant success and survival, this does not mean that osteoporotic patients taking them are to be considered complication-free. One of the most serious (although uncommon) complication that has been associated with the use of antiresorptive medications is the development of osteonecrosis of the jaws (ONJ). ONJ presents as an area of exposed bone that does not heal spontaneously within 8 weeks and it is usually associated with pain (Ruggiero et al., 2014).
Only limited clinical studies have tried to address the risk of ONJ subsequent to implant placement, but it is recommended to consider it comparable to the one associated with a tooth extraction. The incidence of BP-associated ONJ described in the literature ranges from 0.001% to 0.01%, with highest levels for long-term treatments, up to 0.2% in patients with >4 years exposure (Lo et al., 2010). Remarkably,ONJs tend to occur more frequently in patients taking nitrogen-containing BPs (such as zoledronic acid and pamidronate) and intravenous bisphosphonates. Moreover, the literature suggests that patients who undergo surgical trauma during the installation of dental implants may be more susceptible to the development of ONJs (Mendes et al., 2019).
In patients taking denosumab, the risk of developing ONJ can be significantly reduced by planning implant placement after 6 months from the injection (before a new dose is delivered), since this medication does not have residual effects beyond 6 months.
In conclusion, which precautions should clinicians put in place when dealing with osteoporotic patients being treated with antiresorptive medications and requiring an implant-supported rehabilitation? Although a small percentage of patients receiving antiresorptive medications develop ONJ spontaneously, most affected patients develop this complication after dentoalveolar surgeries. Therefore, if systemic conditions allow, initiation of antiresorptive therapy should be delayed until dental health is optimized and all invasive surgeries (including implant surgeries) have been performed. In this respect, close collaboration between dentists and physicians is needed to stress the importance of optimizing dental health throughout this medication-free period. In patients already under treatment, implant placement is not contraindicated, but patients should be adequately informed of the very small risk (<1%) of compromised bone healing.
In order to further reduce the incidence of ONJs, the clinician should firstly identify and address comorbidities and risk factors that may increase the possibility of this serious complication developing, such as smoking, oral mucosal irritations associated with poorly fitting dentures, periodontitis, treatment with corticosteroids and diabetes mellitus. Furthermore, particular care should be taken to minimize surgical trauma, to use abundant irrigation when drilling the bone and to suture in order to promote soft tissue closure for primary intention.
Although there is very limited evidence regarding the effect of treatment duration, the American Association of Oral and Maxillofacial Surgeons suggested that for patients taking BPs for more than 4 years – if systemic conditions permit – a drug holiday might be considered for at least 2 months before and 3 months after elective invasive oral surgeries to reduce the risk of ONJ (Ruggiero et al., 2014). For patients who have taken oral BPs for less than 4 years and have no clinical risk factors, no alteration or delay in the planned surgery is deemed necessary.
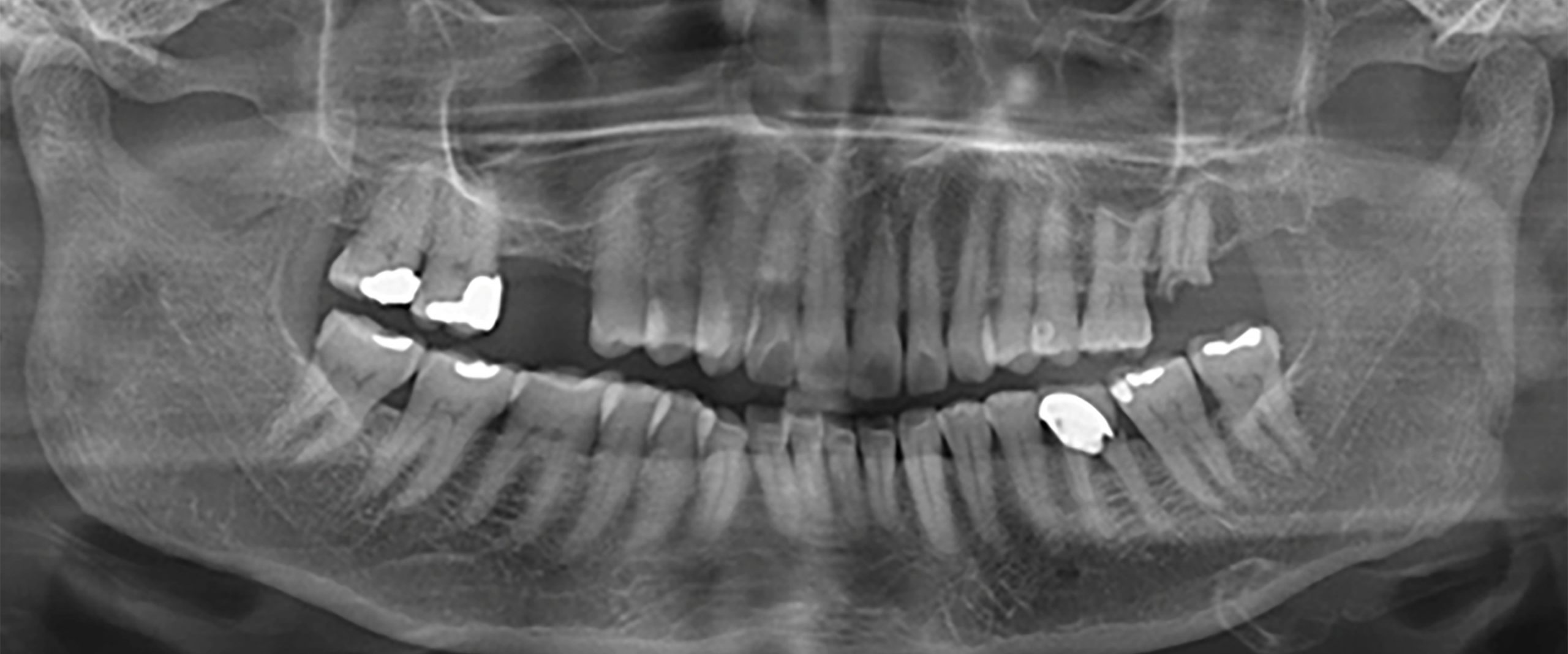
Finally, a regular recall schedule and reinforcement of oral hygiene steps should be particularly stressed in this category of patients. Herein, a case of successful and complication-free management of single implant rehabilitation in a 63-year-old, osteoporotic woman (T-score -2.7 at the hip and lumbar spine) in treatment with denosumab and vitamin D for 2 years is presented (Figs 1 and 2). Three months after atraumatic extraction of lower left six (hopeless due to root fracture), it was decided to rehabilitate the edentulous site with an implant with a hydrophilic micro-rough surface with the aim of enhancing osseointegration. In order to reduce the risk of developing ONJ, implant placement was planned just before the 6-monthly injection of denosumab and under antibiotic coverage. Healing was uneventful and the implant was successfully loaded eight weeks after implant placement.
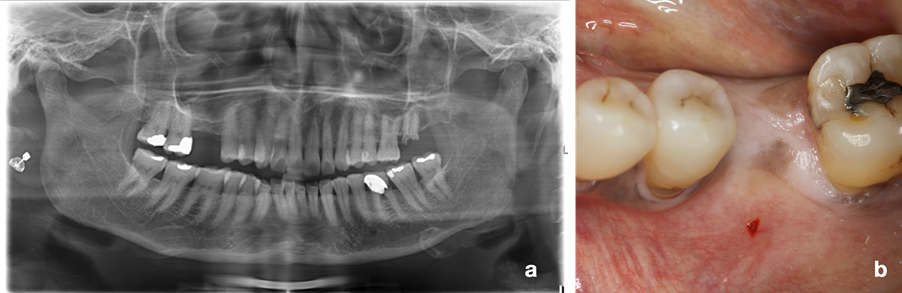
![Fig. 2: An implant with a hydrophilic micro-rough surface was placed in the edentulous site of the lower left first molar (a, b, c, d). Loading was performed 8 weeks after unsubmerged healing (e). At 2 years post-loading, the implant showed a pleasant aesthetic outcome and stable radiographic peri-implant bone levels (f, g, h) [Surgery performed by Dr. Nikos Mardas]](https://blog.iti.org/wp-content/uploads/2021/12/Fig-2.png)