Background
Peri-implantitis is a pathological condition that occurs in the tissues surrounding dental implants. It is characterized by inflammation of the peri-implant connective tissue and progressive loss of supporting bone (Schwarz et al., 2018). A systematic review described a 22% prevalence of peri-implantitis (Derks & Tomasi, 2015). Critical analyses of the literature reported different prevalences between 1% and 47% due to the varying definition of this pathological condition (Tomasi & Derks, 2012). In addition, it has been suggested that this bone loss is time-dependent, and that the follow-up time of the different studies may have affected the prevalence described (Fransson et al., 2015; Derks et al., 2016).
The objective of the treatment of peri-implantitis is to resolve the inflammation of the soft tissues and stop the additional loss of the peri-implant bone. A systematic review reported that, regardless of the non-surgical treatment modality used, it is insufficient to stop the disease (Faggion et al., 2014), while surgical treatment has shown greater efficacy immediately and over the longer term (Romeo et al., 2014; Carcuac et al., 2017). Furthermore, it is demonstrated that factors such as the surface of the implant have a significant influence on the results of surgical treatment (Carcuac et al., 2017; Roccuzzo et al., 2017). The anatomical configuration of the peri-implant bone defect was reported to be another relevant factor, especially when selecting the type of surgical approach (Schwarz et al., 2010)).
The goal of reconstructive procedures for peri-implant bone defects is to restore the implant-supporting tissues (Schwarz et al., 2017; Roos-Jansaker et al., 2014) and thus improve esthetics and achieve a hypothetical re-osseointegration (Persson et al., 2001). The potential benefit of using bone substitutes/biological agents in reconstructive procedures for the treatment of peri-implantitis remains undefined for the time being due to few clinical studies with heterogeneous designs and different follow-up times.
Several studies evaluated the effectiveness of a material without comparing it with a control group, while others either compared different materials or materials with the performance of mechanical debridement only (Aghazadeh et al., 2012; Khoury & Buchmann, 2001; Wohlfahrt et al., 2012). For this reason, it is difficult to draw solid conclusions about the ideal material and therapeutic approach.
The use of enamel matrix derivatives (EMD) has shown excellent results in the regeneration of the attachment of teeth with intra-bony defects and has also been investigated when reconstructing the support bone lost around implants. A randomized clinical trial (Isehed et al., 2016) reported contradictory results regarding the use of EMD in the surgical treatment of peri-implantitis. In addition, another cohort study described the need for better-designed clinical trials to be able to analyze correctly the adjunctive use of EMD with xenografts (Mercado et al., 2018).
The effectiveness of autologous bone for the reconstruction of peri-implant bone resulted in a stable result at 3 years of follow-up (Behneke et al., 2000). On the other hand, satisfactory results have also been reported, leading to a reduction in probing depth of on average 4.23 ± 1.47 mm, with the use of an allograft impregnated in an antibiotic solution (Nart et al, 2017). Titanium granules have been suggested as a graft material; in a multicenter randomized clinical trial their use was compared with performing surgical debridement of the peri-implant lesion only (Jepsen et al, 2016). The primary outcome was the radiographic bone filling and although it is true that statistically significant differences were found in favor of the test group, it is necessary to report the difficulty of distinguishing the biomaterial at the radiographic level. Other studies describe contradictory results regarding the use of this biomaterial (Andersen et al., 2017; Gulet et al., 2017).
One of the most investigated biomaterials in the reconstruction of peri-implant bone defects are xenografts. A recent clinical trial compared their use with autologous bone. The only outcome in which statistically significant differences were described in favor of the xenograft was radiographic bone filling (Aghazadeh et al., 2012). A case series of xenografts for the reconstruction of peri-implant bone defects obtained predictable results in probing depths and radiographic bone filling (Rotenberg et al., 2016). In addition, there was no change in the level of the peri-implant mucosa during the entire follow-up. The use of membranes has shown superior results when combined with bone grafts versus bone grafts alone in terms of bone gain around implants prior to or simultaneously with their placement. Furthermore, the use of EMD improved the osteoconductivity of bone grafts (Froum et al., 2015). Moreover, EMD had an antimicrobial effect and a positive effect on wound healing and tissue regeneration. Nevertheless, there is a lack of scientific evidence to support the use of EMD for the treatment of peri-implant-related intrabony defects.
The objective of the clinical case below is to describe the surgical approach of a reconstructive therapy to treat an intrabony peri-implant defect, using a collagenated xenograft, resorbable collagen membrane and EMD after a peri-implant surface debridement with a chitosan brush. This clinical case is part of an ongoing randomized controlled clinical trial. This study aims to compare the clinical benefit of the adjunctive use of enamel matrix derivative in combination with a xenograft and a collagen membrane in the reconstructive therapy of peri-implant-related intrabony defects.
Clinical case
Initial situation:
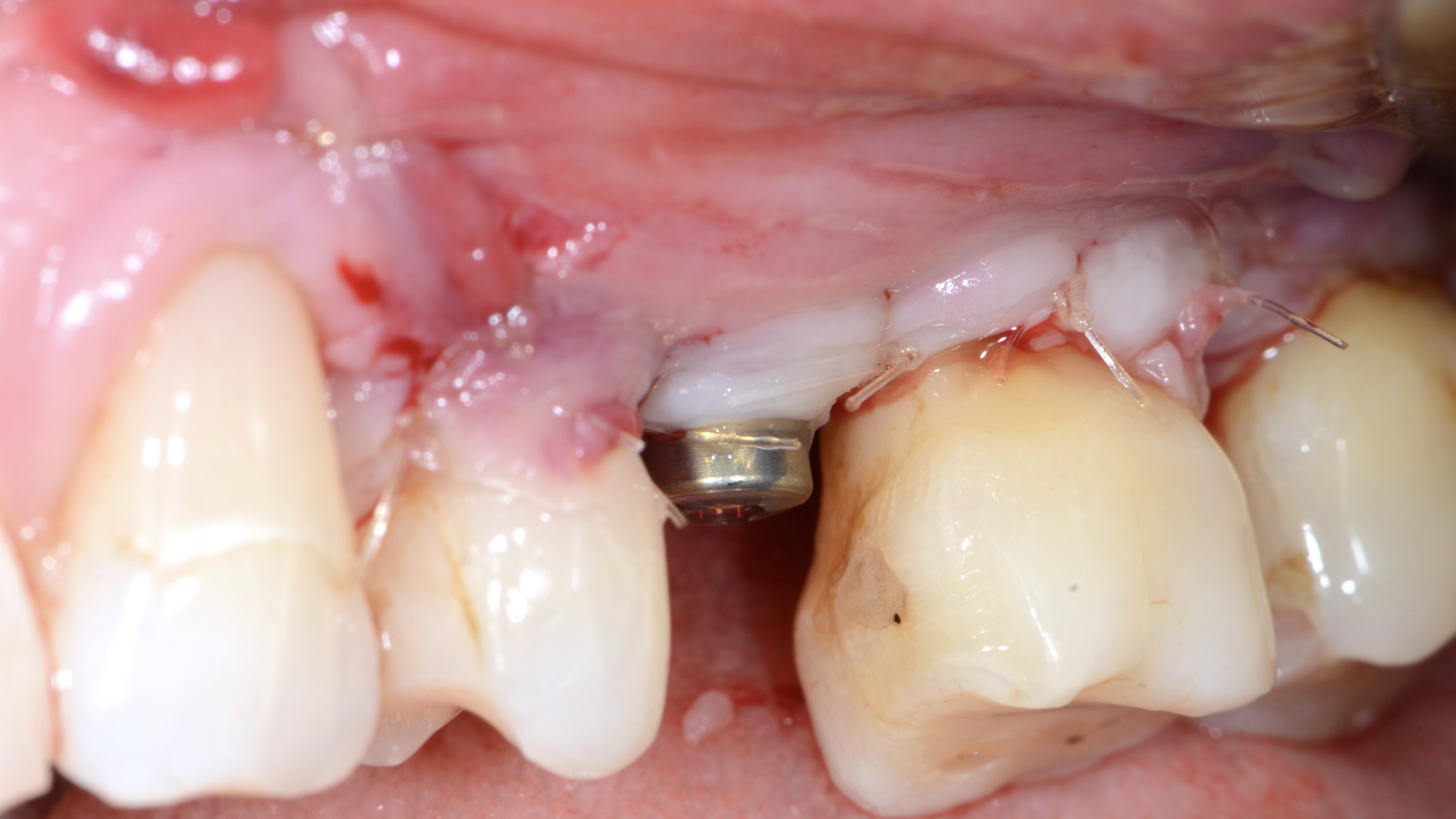
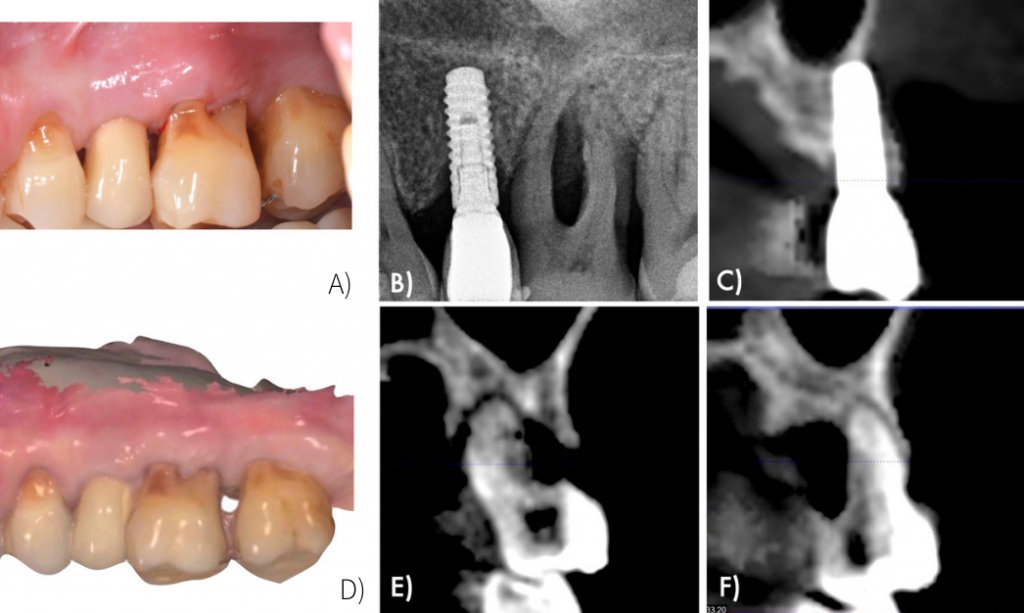
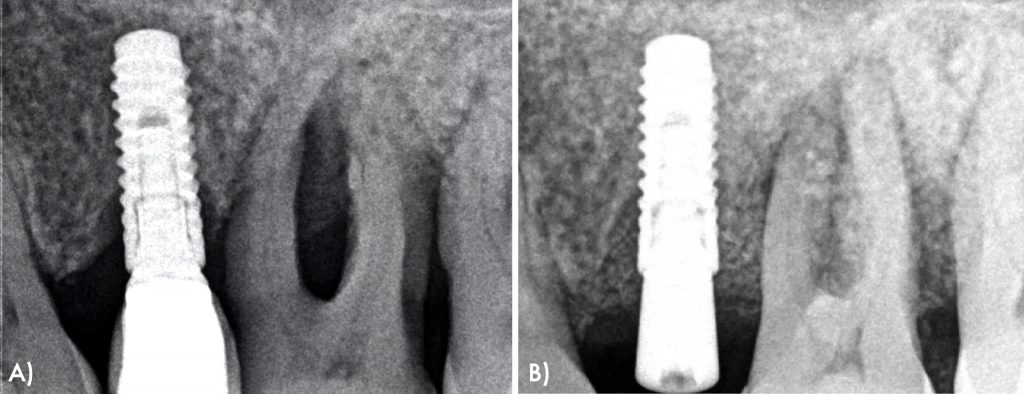
A 62-year-old female presented to a periodontal maintenance visit in 2021. The patient presented bleeding on probing and deep peri-implant and periodontal pockets around the implant #25 and teeth #26 and #27. In addition, in the periapical x-ray, osseous defects with an intrabony component were detected (Figs 1, 2).
Presurgical phase:
The patient received a full-mouth ultrasonic debridement and manual curettage 4 weeks before the surgical protocol was performed. As a result, the patient reached full-mouth plaque and full-mouth bleeding scores below 20% before proceeding with the surgical treatment.
Surgical phase:
The surgical procedure was performed under local anesthesia (Lidocaine HCL 2% with 1:100,000 epinephrine), and the following surgical intervention was carried out after removing the implant-supported prosthesis.
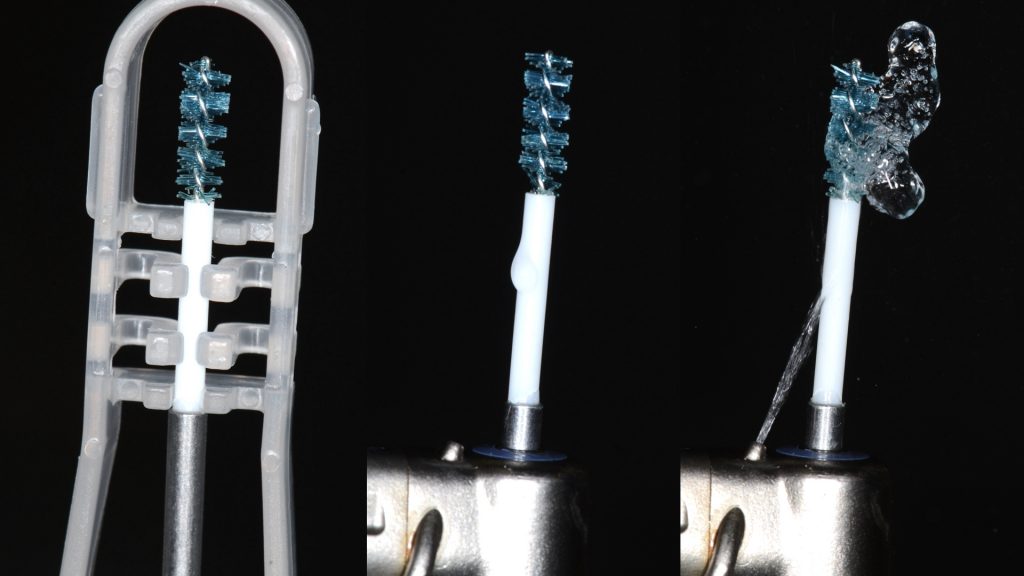
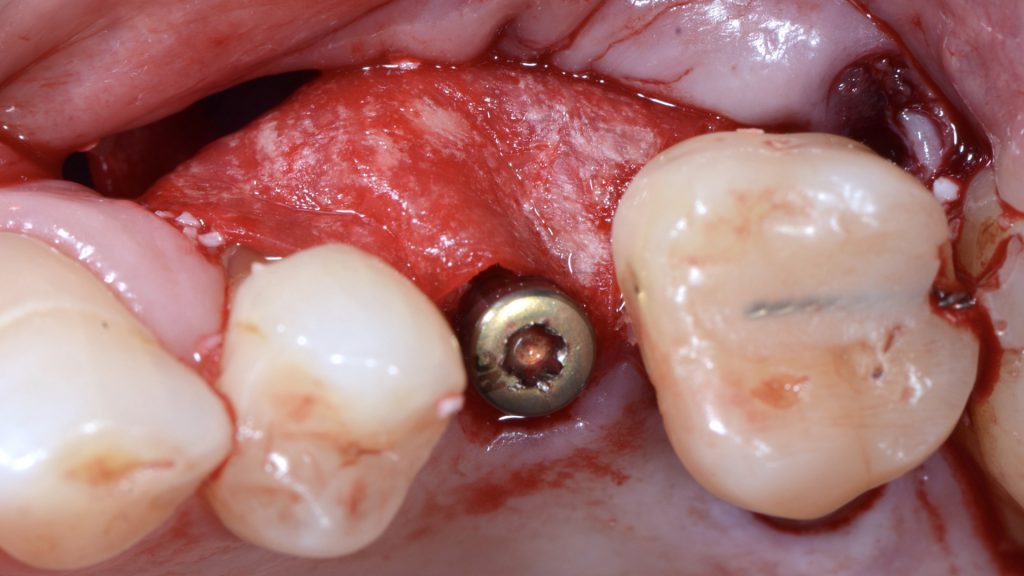
An intra-sulcular incision at the affected implant was made using a 15C blade. The flap was extended mesially, with a beveled vertical releasing incision one tooth away from the affected implant. After buccal full thickness flap elevation and removal of granulation tissue, the exposed implant threads were debrided using a chitosan brush (Labrida BioClean, Straumann, Basel, Switzerland) and ultrasonic debridement was performed around teeth (Figs 3, 4).
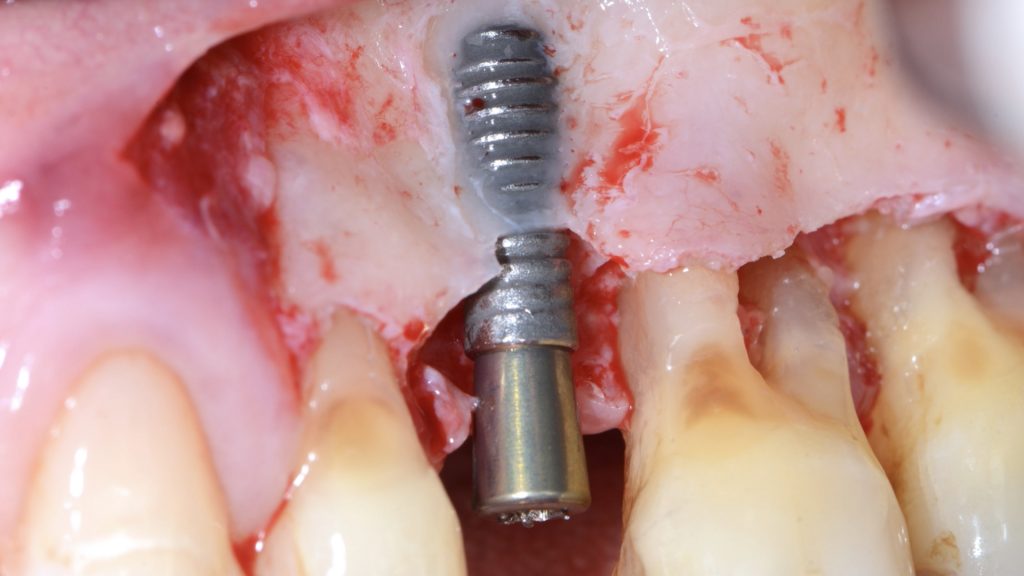
After implant surface decontamination, the implant surface and root surfaces were conditioned with 24% ethylenediaminetetraacetic acid (EDTA, PrefGel Institut Straumann AG, Basel, Switzerland) for 2 minutes. After irrigation of the sites with sterile saline to remove EDTA, a gel containing enamel matrix proteins (Emdogain® Institut Straumann AG, Basel, Switzerland) was applied to the implant and root surfaces (Fig 5).
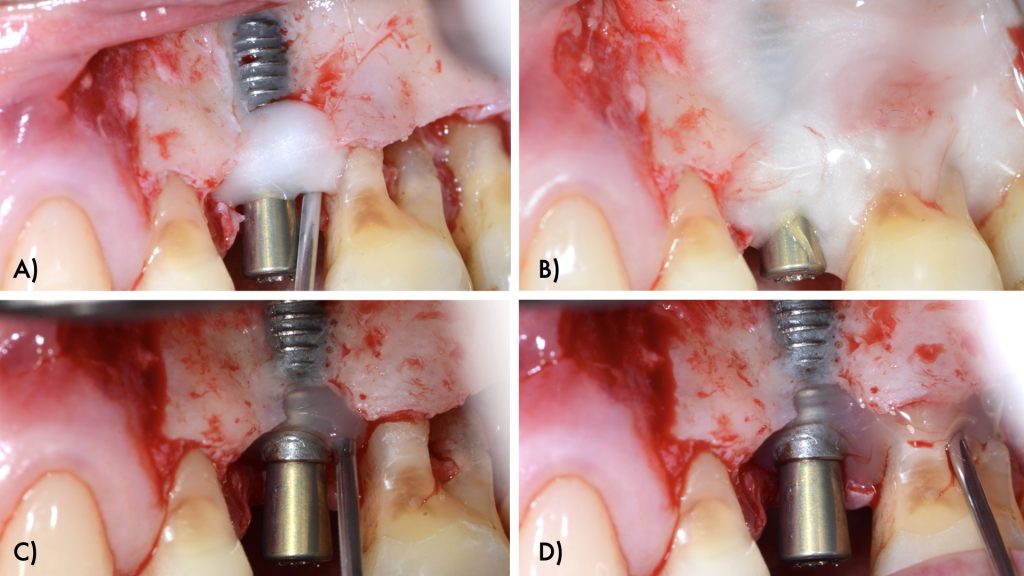
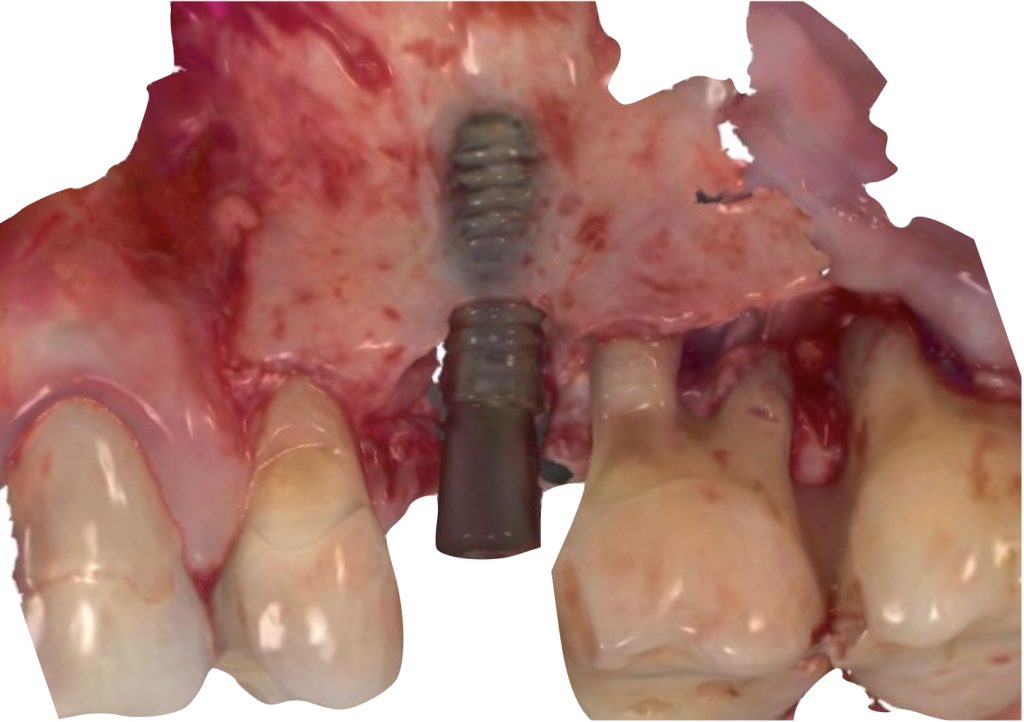
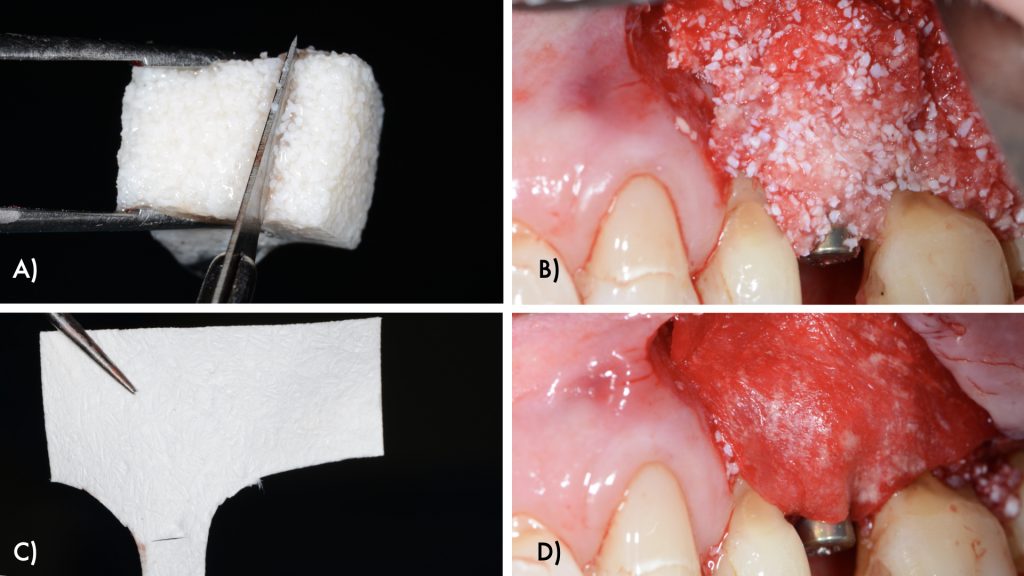
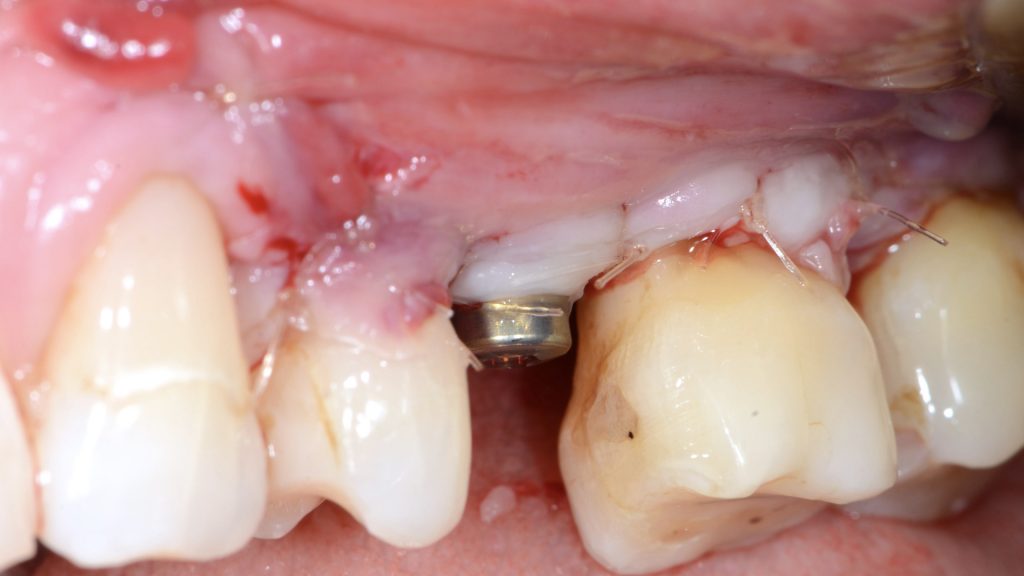
The intraosseous component of the peri-implant and periodontal defects were grafted with a collagenated xenograft (Straumann® XenoFlex, Institut Straumann AG, Basel, Switzerland). The graft was adapted to the defect morphology and covered with a resorbable collagen membrane (Straumann® MembraneFlex Institut Straumann AG, Basel, Switzerland) (Figs 6, 7). Primary wound closure was achieved using internal vertical mattress sutures in mesial and distal aspects, and the gingival recession of 24 was covered, advancing the flap coronally (Figs 8, 9).
Post-operative care
The patient was instructed to apply chorhexidine gel on the area three times a day for two weeks. Furthermore, systemic amoxicillin 500mg (3 tablets a day for 7 days) and anti-inflammatory medication (Enantyum 25mg, 1 tablet every 6-8 hours for 4 days) were also prescribed.
Follow up:
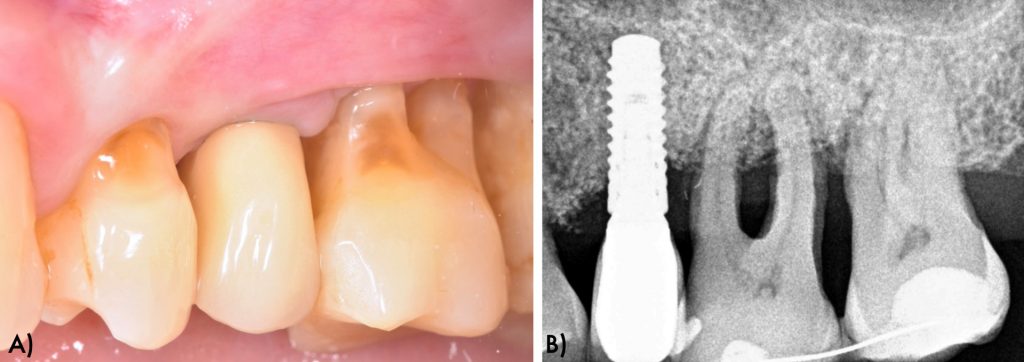
At 6 months follow up visit, patient showed peri-implant health constituted by reduction of probing pocket depths and lack of bleeding on probing clinically and radiographic intrabony defect fill and absence of further bone loss (Figure 10).
Conclusions
- The proposed implant surface decontamination with a chitosan brush appears to be safe and efficient.
- The surgical reconstructive approach using enamel matrix proteins, a collagenated xenograft and resorbable collagen membrane may offer a successful therapeutic option to reconstruct intra-bony peri-implant defects leading to positive effects on the clinical and radiological parameters.
- Randomized clinical trials with longer follow up are needed in order to understand the clinical benefit of adjunctive enamel matrix proteins in the treatment of peri-implant-related intrabony defects.