Introduction
It is estimated that around 12-18 million implants are sold annually across the globe. The likelihood of a patient attending your dental practice with at least one dental implant in situ is high (Klinge et al. 2018). This reinforces the need for general dentists to acquire the knowledge and skills to competently diagnose and appropriately manage disease around implants. This can take the form of peri-implant mucositis or peri-implantitis, both displaying significant prevalence (Derks & Tomasi 2015). Hence the importance of probing around implants is highlighted in this article with appreciation of the subtle differences in anatomy of the surrounding tissues compared to natural teeth. Due to this anatomical variation, peri-implantitis can progress rapidly and unpredictably resulting in complex defect configuration and subsequent management. As a result, prevention of peri-implant diseases is imperative, particularly for high-risk patients. A recently developed Implant Disease Risk Assessment (IDRA) tool is highlighted in this article. This determines a patient’s risk profile in developing peri-implant diseases, which would be of beneficial use to dentists (Heitz-Mayfield et al. 2020).
Peri-implant mucositis
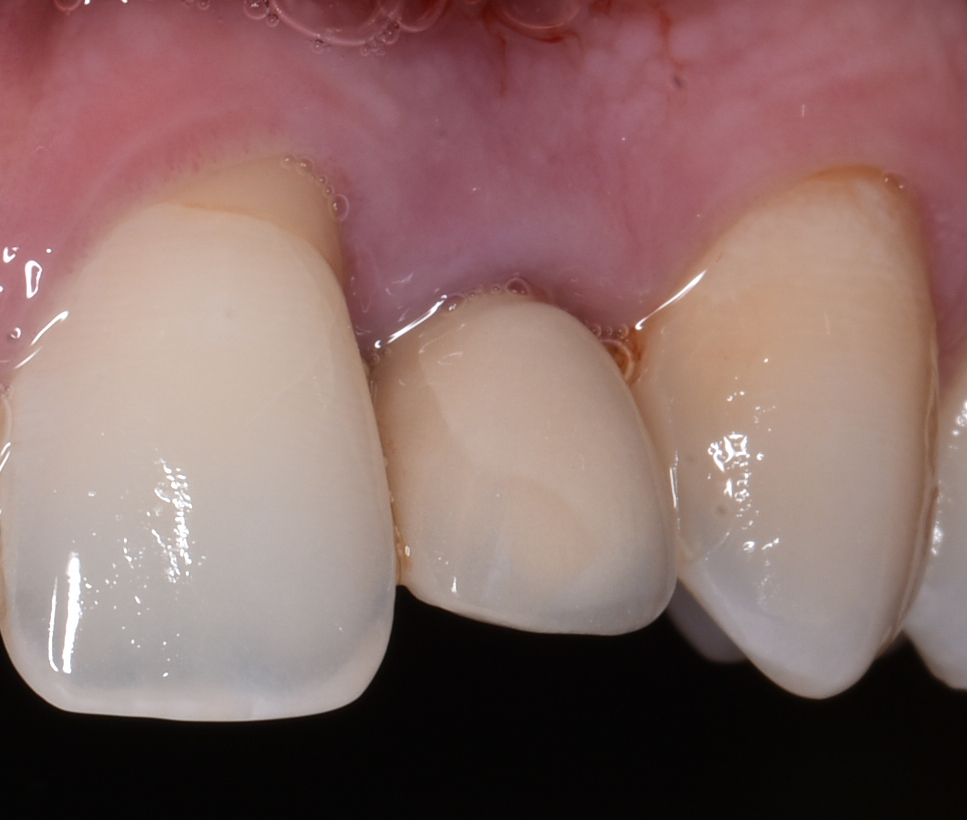
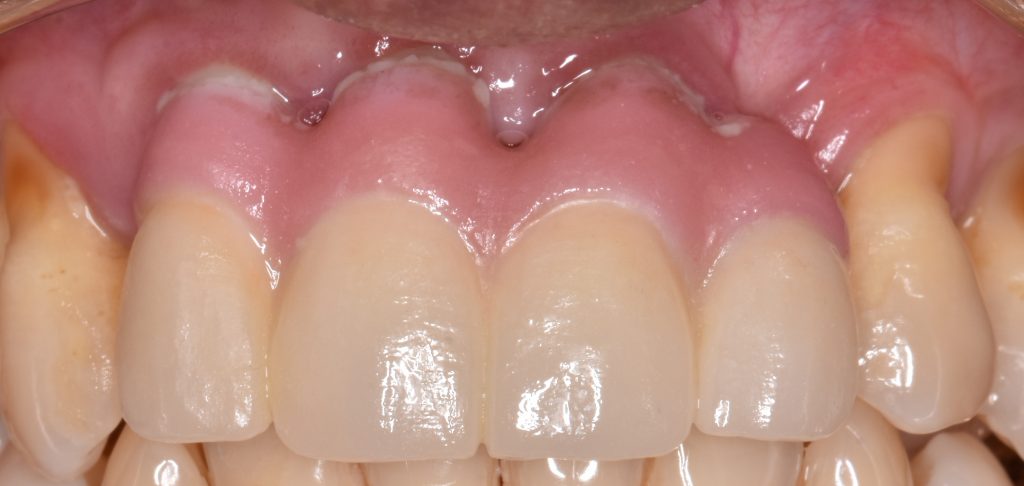
According to ‘The 2017 World Workshop’, peri-implant diseases, in general, can be divided into peri-implant mucositis and peri-implantitis (Berglundh et al. 2018). Peri-implant mucositis is characterized by clinical signs of inflammation around the soft tissues surrounding a dental implant without signs of progressive marginal bone loss of more than 2 mm after supra-structure installation (Figs 1a and b).
Peri-implantitis
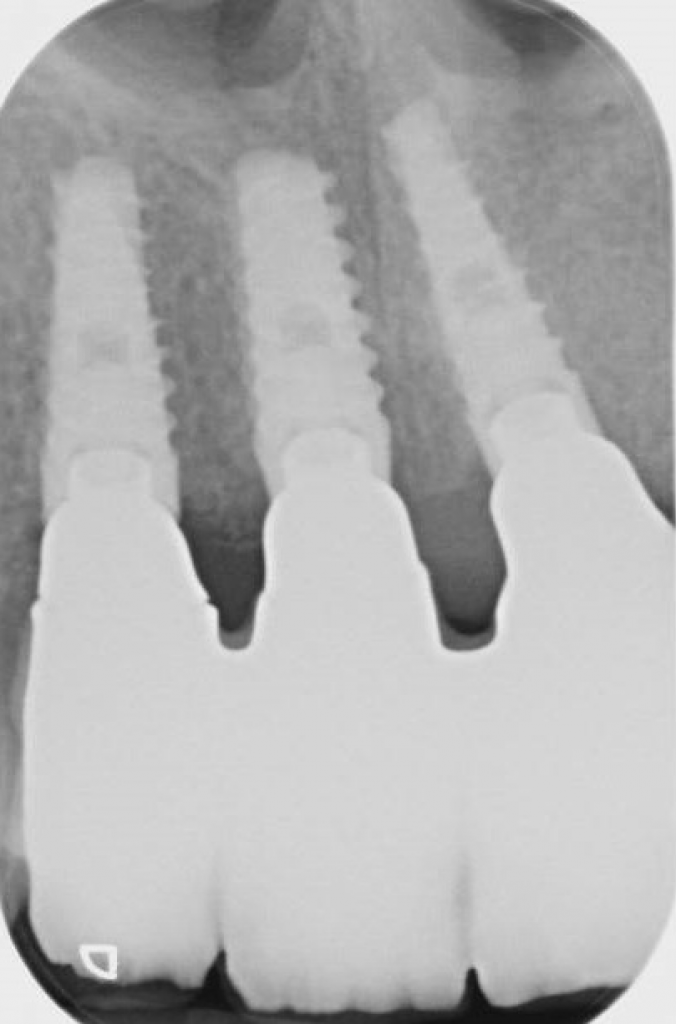
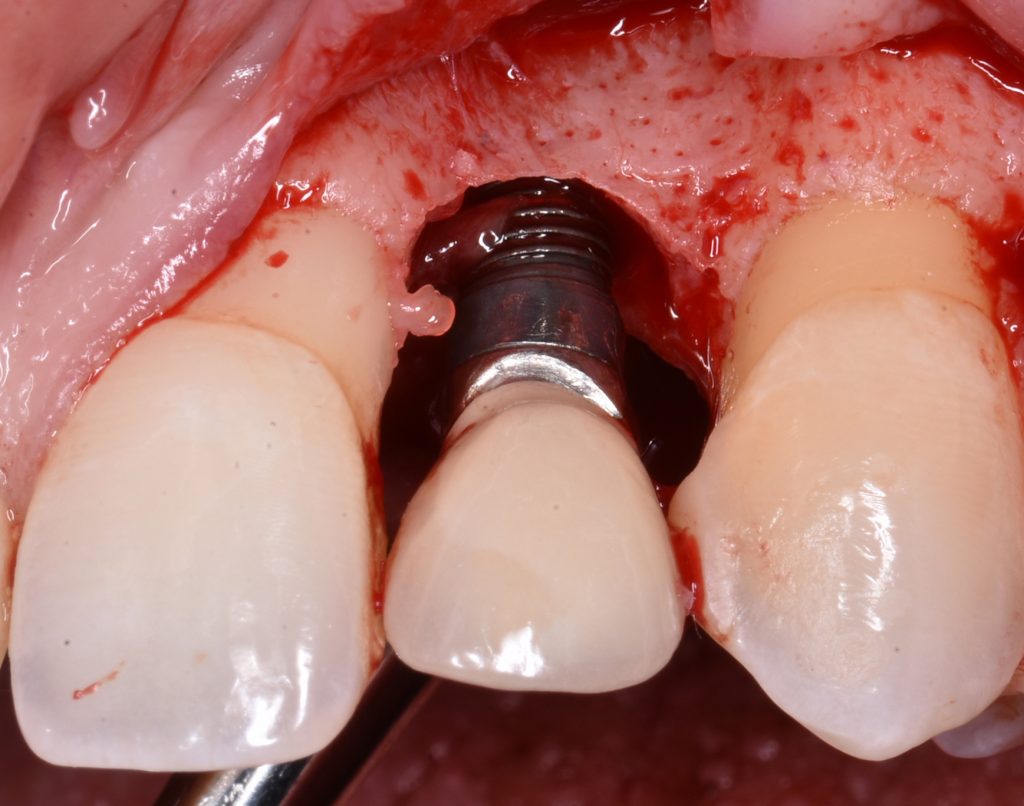
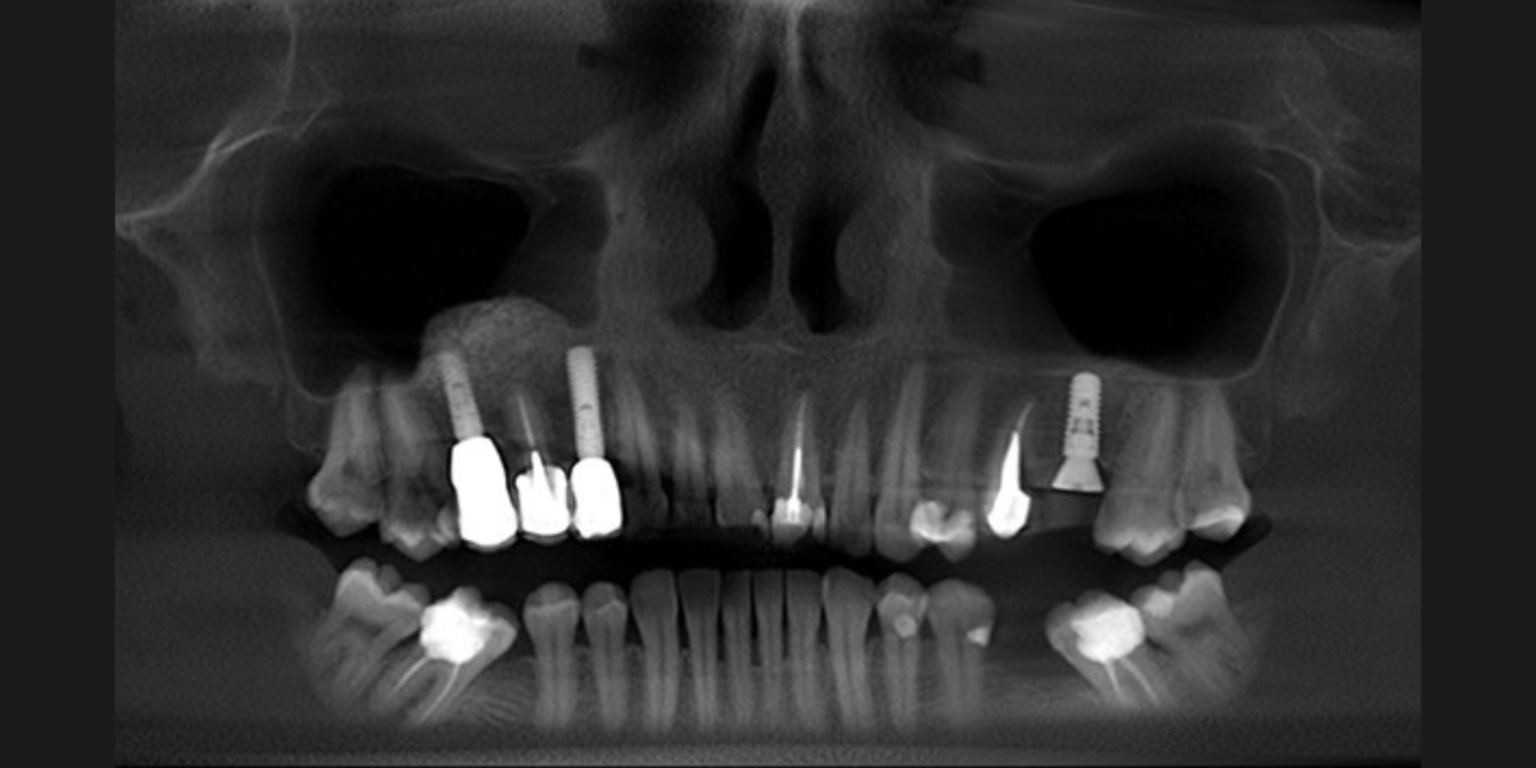
Peri-implantitis is characterized by inflammation around the peri-implant tissues, typically with deepening of probing pocket depths concomitantly with progressive supporting bone loss (more than 2 mm) beyond the initial bone remodeling phase after prosthesis installation (Figs 2 and 3). In the absence of previous clinical and/or radiographic records, peri-implantitis can be defined as the combination of ≥3 mm bone loss from the crestal bone level and ≥6 mm probing depths with bleeding or suppuration (Berglundh et al. 2018).
Classification of peri-implant bone defects
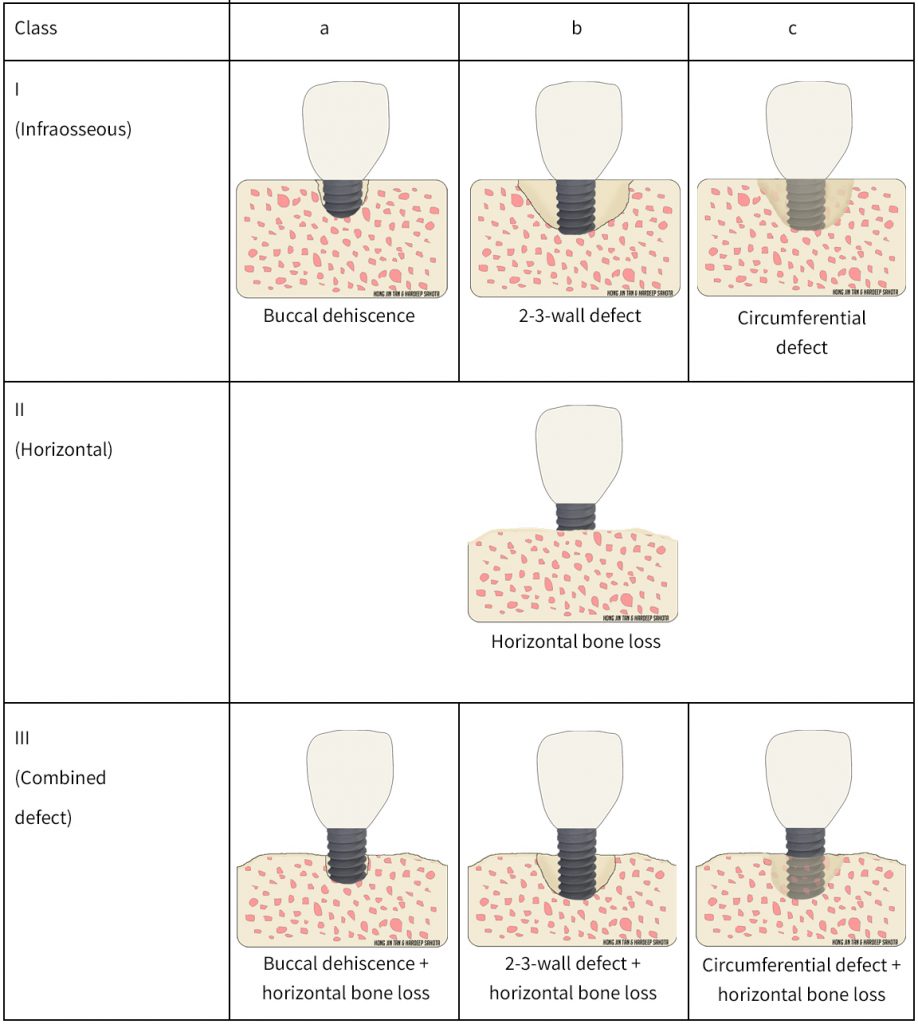
Classifying peri-implant bone defects is key to ultimately aiding communication and treatment planning between colleagues. Various peri-implant bone defect classifications have been proposed. Recently, Monje and co-workers proposed a classification that divides peri-implant bone loss into different defect morphologies and severities (Monje et al. 2019). Peri-implant bone loss was classified into class I: infraosseous, Class II: horizontal, and Class III: combination defects. Infraosseous defects are further subclassified into a: dehiscence, b: 2/3-wall, and c: circumferential. The amount of bone loss in relation to the implant neck was used to categorize the severity; Grade S: slight, Grade M: moderate, and Grade A: advanced (Monje et al. 2019). Please refer to tables 1 and 2 for diagrammatic illustrations. Based on CBCT analyses, the most common defect morphology and severity was class Ib and moderate severity, respectively. It is important to note that early evidence suggests different defect anatomy may inform treatment modalities, however further studies are warranted (Schwarz et al. 2010). It is not our intention in this article to discuss treatment approaches for peri-implant diseases.
| Severity | Amount of bone loss | Bone loss to implant length ratio |
| Grade S (Slight) | 3-4 mm | <25% |
| Grade M (Moderate) | 4-5 mm | ≥25-50% |
| Grade A (Advanced) | 6 mm | >50% |
Anatomy and histology
Peri-implant mucositis shares numerous similarities with gingivitis, as both are preventable and reversible, albeit the former may take slightly longer to resolve (Heitz-Mayfield & Salvi 2018). Although peri-implantitis and periodontitis both have a comparable phenotype, the pathogenic mechanism of peri-implantitis is still unclear. It is generally accepted that the etiology of peri-implant diseases is disruption of the host-microbe homeostasis. Disease progression rates for periodontitis and peri-implantitis differ, the latter often progressing more rapidly (Schwarz et al. 2018). The high disease progression rate around implants may be explained by multiple factors, such as the lack of periodontal ligaments inserting into the implant, larger inflammatory lesions around the peri-implant mucosa, and the lack of a “self-limiting” ability to separate the inflammation from the bone as compared to gingival tissues around natural teeth (Berglundh et al. 1991; Berglundh et al. 2011; Schou et al. 1993).
Around teeth, the supra-crestal connective tissue attachment is typically around 3 mm. This consists of the gingival sulcus (1.32 mm), junctional epithelium (1.14 mm) and the connective tissue (0.77 mm) (Vacek et al. 1994). The orientation of the fibers within this compartment is vast providing support of the gingivae to the tooth; the main types being the dentogingival, dentoperiosteal, circular and transseptal fibers. The connective tissue is composed of 60% collagen fibers (predominantly type I collagen) and around 5-15% fibroblasts (Vacek et al. 1994). Further structural support is provided by the periodontal ligament (PDL), which attaches the tooth to the alveolar bone. Similarly, the orientation of the PDL fibers include horizontal, oblique, apical and alveolar crest fibers.
In contrast, the peri-implant mucosa dimension may vary based on the implant type. Submerged implants (2-stage) demonstrated a sulcus and junctional epithelium of 2.14 mm combined and connective tissue of around 1.66 mm (Berglundh et al. 1991). For unsubmerged implants (1-stage), the sulcus and junctional epithelium combined are 1.96 mm, whilst the connective tissue compartment is around 1.05 mm (Cochran et al. 1997). On the other hand, Abrahamsson and co-workers demonstrated a similar dimension of the peri-implant mucosa irrespective of the type of implant installation procedure; 2 mm of sulcus and junctional epithelium and 1.5 mm of connective tissue (Abrahamsson et al. 1996; Abrahamsson et al. 1999). Around implants, the connective tissue is composed of 85% parallel-oriented collagen fibers and 1-3% fibroblasts. This less vascularized and highly fibrous tissue is analogous to scar tissue; referred to as ‘inflammation-free scar tissue’ by Buser and colleagues (Buser et al. 1992).
Experimental peri-implantitis studies on animals have demonstrated greater bone destruction around implants than teeth, as well as a larger inflammatory cell infiltrate within the connective tissue. This inflammatory lesion extended apical of the pocket epithelium and reached the bone crest around implants and did not show the same ‘self-limiting’ process that occurs around teeth (Lindhe et al. 1992; Marinello et al. 1995; Schou et al. 1993).
Prevalence of peri-implant diseases
The prevalence of peri-implant mucositis and peri-implantitis has been recently reported at around 43% and 22%, respectively (Derks & Tomasi 2015). The high prevalence is concerning as the most effective treatment for peri-implantitis has yet to be identified (Roccuzzo et al. 2018). Peri-implant mucositis is assumed to precede peri-implantitis, but it may persist without ultimate progression to peri-implantitis. The successful treatment of peri-implantitis is unpredictable with studies demonstrating a wide range of success in the short term (12 months) as well as recurrence of disease, implant loss and progression of bone loss also being reported (Heitz-Mayfield & Mombelli 2014). A recent systematic review showed implant survival rates ranging from 76 to 100% at 5 years following treatment of peri-implantitis, when a personalized supportive care regime was included (Heitz-Mayfield et al. 2018). The success rates often defined as no further bone loss, no deep probing depths, no bleeding on probing, and no suppuration were generally lower than the survival rates (Roccuzzo et al. 2018). Additionally, mere retention of an implant after treatment of peri-implantitis may not be considered as treatment success for patients due to possible esthetic complications and high associated costs. Therefore, it is essential to reduce the risks of developing peri-implantitis from the outset.
Probing
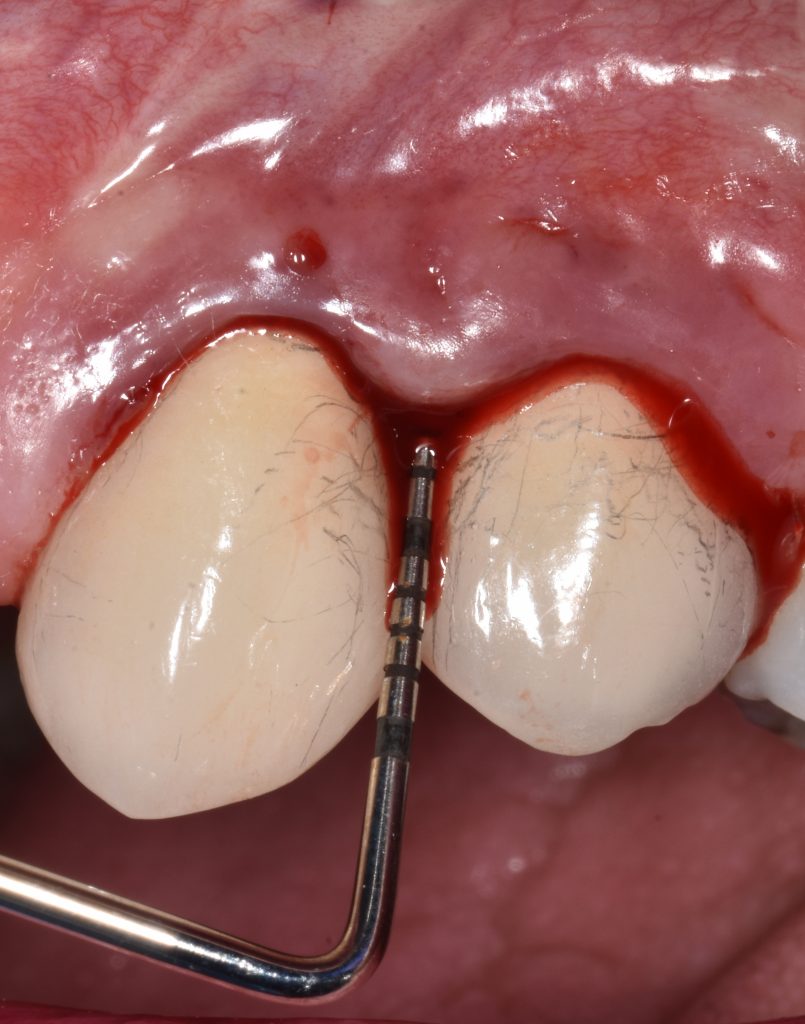
The subtle differences in anatomy, as highlighted above, lend themselves to differences in probing resistance. As a result, healthy gingiva is more resistant to probing forces compared to healthy peri-implant mucosa (Mombelli et al. 1997). In general, the penetration of the probe is greater at implant sites than teeth with more frequent bleeding on probing and lateral displacement of tissues (Ericsson & Lindhe 1993). This is similarly the case in peri-implantitis lesions where the probe penetration is greater and hence the distance between the probe and bone is decreased compared with periodontal inflamed tissues (Lang et al. 1994; Schou et al. 2002). There is no clear threshold for probing pocket depth (PPD) that defines health or disease around implants as appreciation of clinical and radiographic findings are of paramount importance. For example, a PPD of 6 mm around an implant with no sign of bone loss and no bleeding on probing can be defined as healthy. The consensus, as for teeth, is a probing force of 0.25N around implants, which does not damage the peri-implant tissues (Heitz-Mayfield 2008). Therefore, it is recommended to probe around implants to assess bleeding, PPD changes, recession and/or suppuration.
Implant disease risk assessment
As mentioned above, peri-implantitis is a considerable biological complication with unpredictable progression rates. Therefore, prevention is key to reducing the risk of peri-implant diseases. Recently, an Implant Disease Risk Assessment (IDRA) tool was developed by Heitz-Mayfield and co-workers to determine a patient’s risk profile in developing peri-implant diseases (Heitz-Mayfield et al. 2020). 8 parameters are proposed, each with their own set of criteria categorizing low, medium, or high risk (refer to table 3 for full description). As a result, the tool determines the patient’s overall risk profile. In general, a high-risk patient is categorized as having at least 2 parameters in the high-risk category. Whereas a low-risk patient has all parameters in the low-risk category or at most one parameter categorized as moderate risk (Heitz-Mayfield et al. 2020). The tool is useful in guiding clinicians on the need to modify higher risk parameters. It is important to note that one non-modifiable parameter is a history of periodontitis. Therefore, identifying this cohort of patients with implants is crucial, as the criteria to be labelled low risk are more stringent. To inform readers, this is a recently developed tool, and would suggest reference for further details (Heitz-Mayfield et al. 2020).
| Parameter | Low risk | Moderate risk | High risk |
| History of periodontitis | No | – | Yes |
| Percentage of sites with BOP | <10% | 10-25% | 25% |
| Prevalence of probing depth with ≥5 mm | ≤2 sites | 3-6 sites | 6 sites |
| Periodontal bone loss in relation to age | <0.5 | 0.5 to <1 | ≥1 |
| Periodontitis susceptibility | – Stage 1, Grade A | – Stage 2 or 3 – Grade A or B | – Stage 4 – Any Grade C |
| Supportive periodontal therapy | Compliant to recommended recall interval | ≤5 months | ≥6 months |
| Distance from the restorative margin of the implant-supported prosthesis to the bone | Tissue-level implant | 1.5 mm | ≤1.5 mm |
| Implant prosthesis-related factors | Cleansable, well-fitting, screw-retained, or no excess cement | Poor fit with supragingival margin | Not cleansable, poor fit with subgingival margin |
Conclusion
With the ever-increasing demand for implant placement to restore edentulous sites, the prevalence of peri-implant diseases is expected to increase. The first step in appropriately managing such diseases is to correctly diagnose peri-implant health, peri-implant mucositis or peri-implantitis. This is of particular significance in periodontitis patients where the risk of peri-implant disease is substantially higher (Roccuzzo et al. 2010; Schwarz et al. 2018). Diagnosis is attained through probing, appreciation of clinical signs such as bleeding and/or suppuration and radiographic assessment. Additionally, use of the IDRA tool will guide clinicians in highlighting higher risk patients who would require more stringent and closer follow-up.
Take home messages
- Peri-implant mucositis is characterized by inflammation around the soft tissues
- Peri-implantitis is characterized by inflammation and bone loss
- 1 in 5 patients develop peri-implantitis
- Probing is recommended to diagnose and detect peri-implant diseases; prevention is key
- Supracrestal tissue attachment is different around implants compared to natural teeth
- IDRA is a useful risk assessment tool for patients with implants
More from the authors:
More on the topic: