Abstract
The recently published ITI Treatment Guide Vol. 13: Prevention and Management of Peri-implant Diseases comprehensively presents diagnostic- and treatment-related aspects of peri-implant mucositis and peri-implantitis. Peri-implant mucositis and peri-implantitis are caused by biofilm accumulation and dysbiosis, thus decontamination of the implant/suprastructure construct is essential to re-establish peri-implant health. Non-surgical therapy of peri-implantitis is often ineffective in achieving adequate implant surface decontamination and in controlling the disease and therefore surgery is commonly needed to get proper access to the implant surface. Various surgical treatment approaches are available, ranging from open flap debridement to resective or reconstructive surgery. A frequent sequela of peri-implantitis itself, but also of surgery, is the exposure of the implant surface to the oral environment. Implants with exposed modified surfaces are challenging to keep clean. Therefore, in cases where this is expected to occur after surgery, removal the implant threads and smoothening of the modified implant surface, a procedure coined as implantoplasty, have been suggested with the aim of facilitating better biofilm control post-operatively. Although the necessity of implantoplasty is still controversial, no remarkable complications have been reported in the literature. Thus, it is worthwhile to take a closer look at this adjunctive procedure to surgical peri-implantitis therapy.
Background
Peri-implant mucositis and peri-implantitis are biological complications of dental implants caused by a dysbiotic biofilm on the implant surface and prosthetic reconstruction components. Peri-implant mucositis is characterized by the presence of clinical signs of inflammation in the soft peri-implant tissues, but there is no peri-implant bone loss. More specifically, it is characterized by bleeding on gentle probing, swelling, and erythema; suppuration and deepened probing pocket depths may be present, too (Heitz-Mayfieldet al. 2018; Berglundhet al. 2018). Peri-implantitis is characterized – in addition to these inflammatory signs – by the presence of progressive peri-implant bone loss (Berglundh et al. 2018; Schwarzet al. 2018).
Once the diagnosis of peri-implantitis is established, therapeutic strategies aim to resolve the inflammation (Heitz-Mayfieldet al. 2014) and, if possible, to reconstruct the intra-osseous aspects of the defects (Jepsenet al. 2016). First, efforts should be made to focus on guiding the patient in adequate daily biofilm control (Ponset al. 2021). This includes assuring that the prosthetic design allows proper access to clean the entire periphery of the implant emergence; if this is not the case, the implant prosthesis should be modified accordingly (Cortelliniet al. 2019). This hygienic phase should be combined with non-surgical decontamination procedures. Although non-surgical therapy is often ineffective in achieving adequate implant surface disinfection and in disease resolution, it should always precede any surgical treatment (Cosgarea et al. 2023; Dos Santos Martinset al. 2022; De Waal et al. 2022; Ramanauskaite et al. 2019; Heitz-Mayfield et al. 2014). This is suggested in order to have less inflamed peri-implant soft-tissues during surgery, thus allowing for better handling, and also to have the wound environment at a stage towards inflammation resolution. After early re-evaluation of this first non-surgical treatment step, surgical therapy is frequently indicated. A surgical approach allows for better access to the implant surface, more efficient disinfection, and either resective (e.g., osteoplasty, gingivectomy) or reconstructive approaches. Thereafter, frequent supportive implant care is the key to long-term success (Klingeet al. 2018; Roccuzzo et al. 2018; Monje et al. 2016).
For a comprehensive overview, please see the recently published ITI Treatment Guide Volume 13: Prevention and Management of Peri-Implant Diseases (Heitz-Mayfield & Salvi 2022), which presents diagnostic- and treatment-related aspects of peri-implant mucositis and peri-implantitis.
Depending on the manufacturer, currently commercially available dental implant systems have threads of different designs. Additionally, treatment of the originally turned implant surface (e.g., sand blasting and acid etching) is commonly used to achieve a modified, structured, (moderately) rough surface to enhance osseointegration and bone-to-implant contact (Smeetset al. 2016).
As a consequence of the bone loss occurring during peri-implantitis and its treatment, the threaded and modified implant surface might be exposed to the oral cavity and come into contact with the peri-implant mucosa (Sanz-Martínet al. 2021). Exposure of the modified implant surface often impedes adequate oral hygiene and thereby contributes to plaque/biofilm accumulation, dysbiosis, and disease re-occurrence. Therefore, implantoplasty has been proposed as an adjunctive measure in the surgical treatment of peri-implantitis. Implantoplasty includes mechanical removal of the threads and the modified implant surface aiming to achieve a smoother surface (Lozadaet al. 1990). Thereby one can achieve complete implant surface decontamination (El Chaaret al. 2020; Geremiaset al. 2017) for better postoperative biofilm control (Zaugget al. 2017). A recent in vivo proof-of-concept study (Azzolaet al. 2020) indicated that early biofilm accumulation is significantly slower on implant surfaces treated with implantoplasty compared to modified (rougher) surfaces not treated with implantoplasty.
Peri-implantitis induced marginal peri-implant bone loss results in different defect morphologies (Wehneret al. 2021; Monjeet al. 2019; Schwarzet al. 2007), which determine the choice of the surgical treatment approach (Schwarzet al. 2010; Schwarzet al. 2013). Implantoplasty is recommended for those defects and/or defect aspects where bone regeneration cannot be expected, such as supra-osseous defects and buccal or lingual dehiscences. In the first comparative study (Romeoet al. 2005; Romeoet al. 2007) on the effect of implantoplasty as an adjunct to resective surgery, the authors reported stability of probing attachment and peri-implant marginal bone levels during a 3-year follow-up period, while the control group treated by resective surgery without implantoplasty was terminated after 2 years due to progressive peri-implant marginal bone loss. This positive effect of implantoplasty as an adjunct to resective surgery was confirmed in a couple of case-series (Bianchiniet al. 2019; Monjeet al. 2022). For example, during a follow-up period of 24 to 73 months, stable peri-implant marginal bone levels, resolution of suppuration, low prevalence of bleeding on probing of 10.7%, and an implant survival rate of 87% were reported (Bianchini et al., 2019).
As mentioned above, a second indication for implantoplasty is buccal and/or lingual dehiscences, which are often combined with intra-osseous defects. While the intra-osseous defects may receive reconstructive therapy, the buccal and/or lingual dehiscences are treated with implantoplasty. i.e., with a “combined approach”(Monje& Schwarz 2022; Schwarzet al. 2017; Schwarzet al. 2014). Successful results have been reported with this combined approach even in the long-term. For example, irrespective of the decontamination method applied, 15 out of 32 patients treated with a combined approach demonstrated low bleeding on probing values (≤10%) and shallow probing pocket depths (≤ 4 mm) 7 years after surgery. Of the remaining patients, only 4 showed progressive bone loss, while 13 were unreachable (Schwarz et al. 2017).
Nevertheless, implantoplasty is controversially discussed, with claims that it may increase the risk of implant fracture due to diminished implant strength or that the unavoidable deposition of titanium particles in the surrounding tissues may lead to inflammation. Based on a systematic review of the available literature on implantoplasty (Stavropouloset al. 2019), these concerns are, however, not confirmed. More specifically, among 18 clinical studies including implantoplasty as part of the surgical treatment of peri-implantitis and available at that time, no single implant fracture was reported; only a single case of metal-like-mucosal pigmentation was mentioned (Schwarzet al. 2011). Further, although laboratory studies confirmed diminished implant strength after implantoplasty, this might only be clinically relevant in certain situations with narrow diameter implants (Bertlet al. 2021).
Case 1 – Resective surgical approach
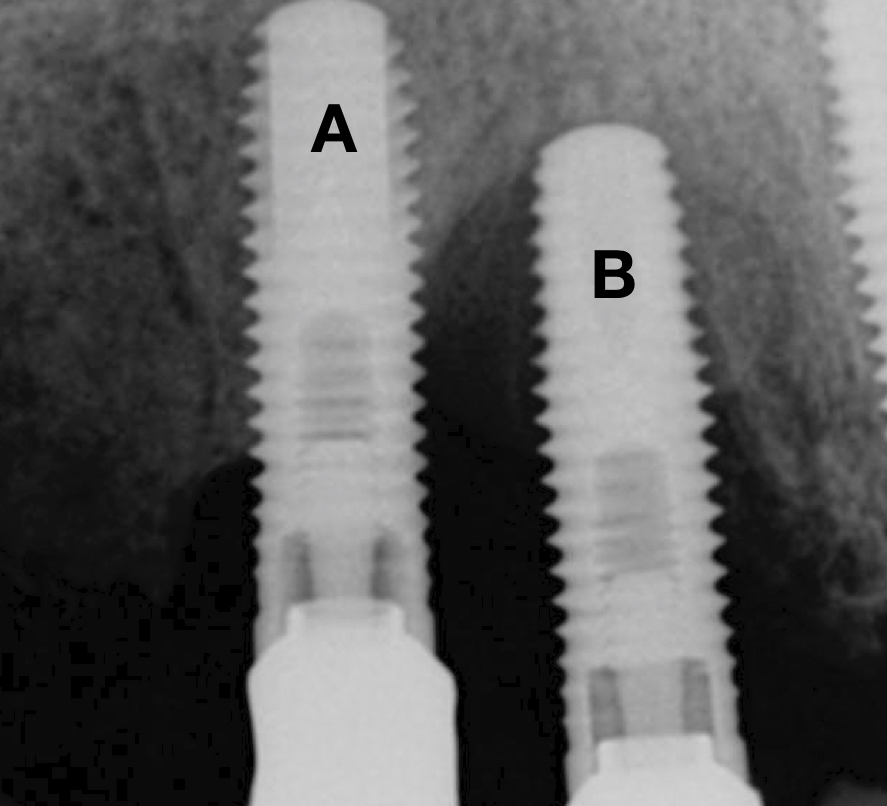
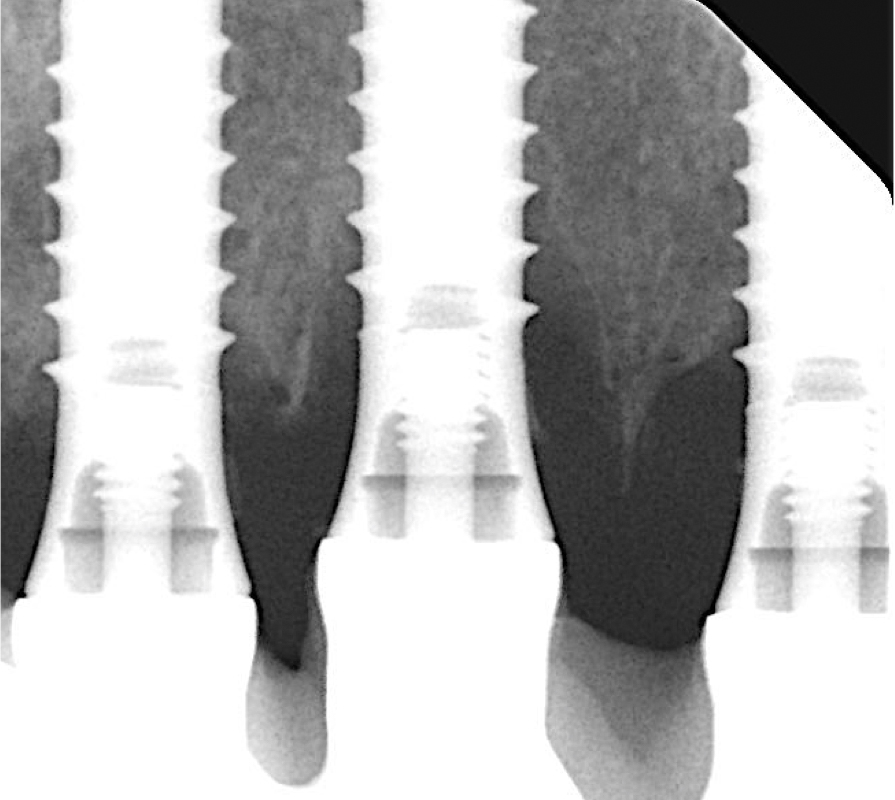
The following two cases were treated at the University Malmö Dental Clinic. The decision-making process of treating or explanting an implant affected by peri-implantitis is influenced by the percentage of bone loss (Figs 1 and 2), the strategic position of the implant, the type of supraconstruction, and/or the esthetic demands of the patient (Heitz-Mayfield & Salvi 2022).
A thorough analysis of the smile line and clear communication with the patient are important to manage expectations, since resective treatment approaches are associated with significant mucosal recession (Romeo et al. 2005; Sanz-Martín et al. 2021). While the mesial implant in this specific case was deemed irrational to treat (Figs 1 and 2) the distal implant (Fig. 1) was scheduled for non-surgical therapy, followed by a resective treatment approach, which would make it possible to meet the patient’s expectations, i.e., keeping the current prosthetic solution (full-arch fixed prosthesis) despite its esthetic limitations.
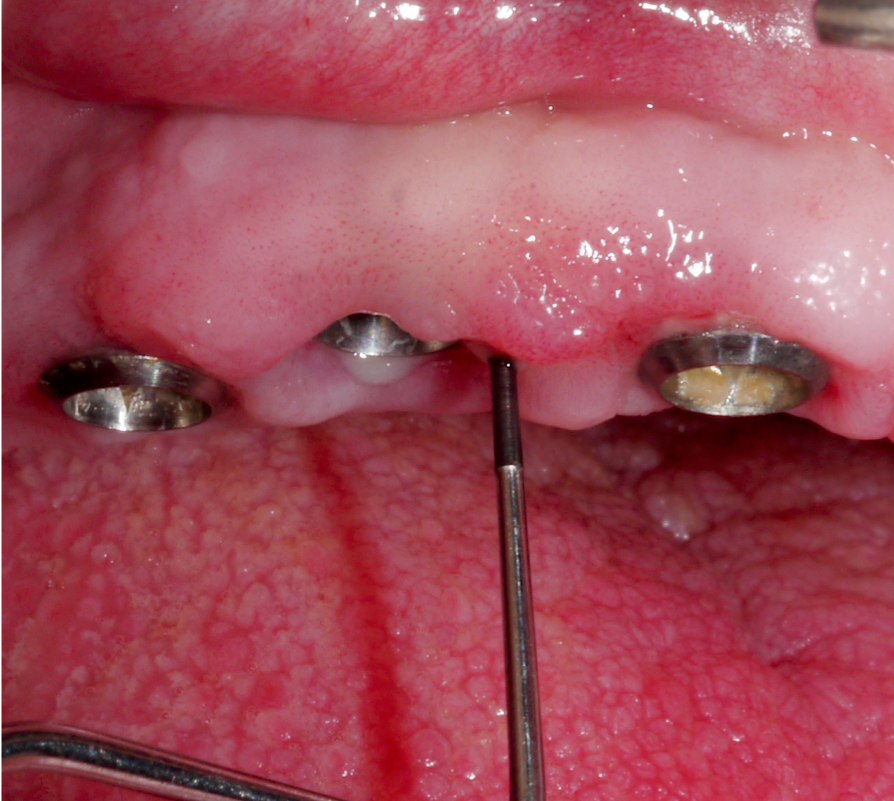
Whenever possible, the supraconstruction should be removed for proper assessment of the case, treatment planning, and – most importantly – improved access during non-surgical and surgical therapy (Heitz-Mayfield & Mombelli 2014) (Fig. 3).
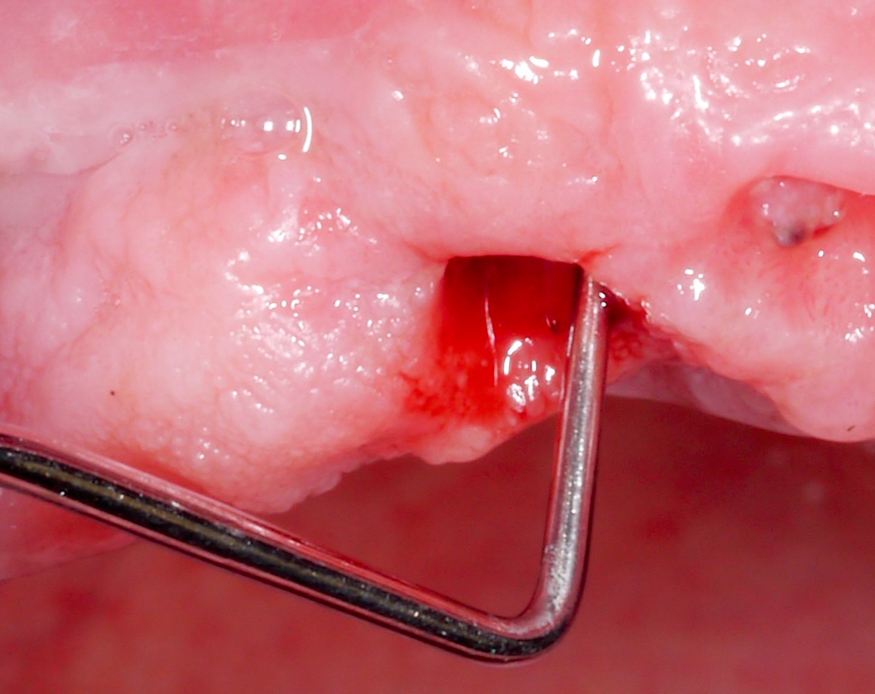
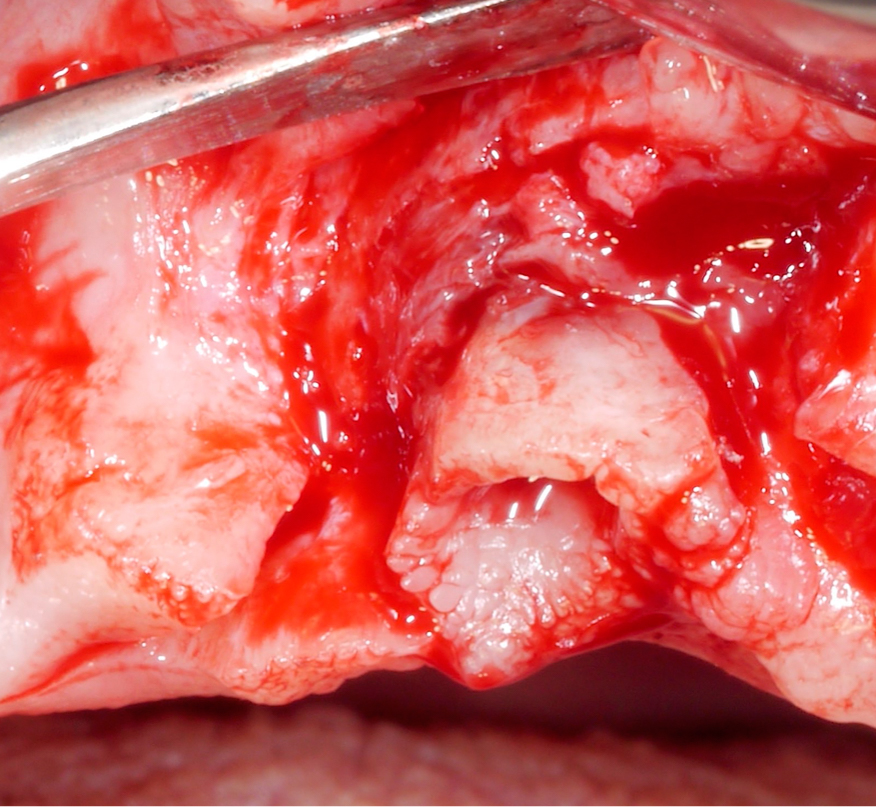
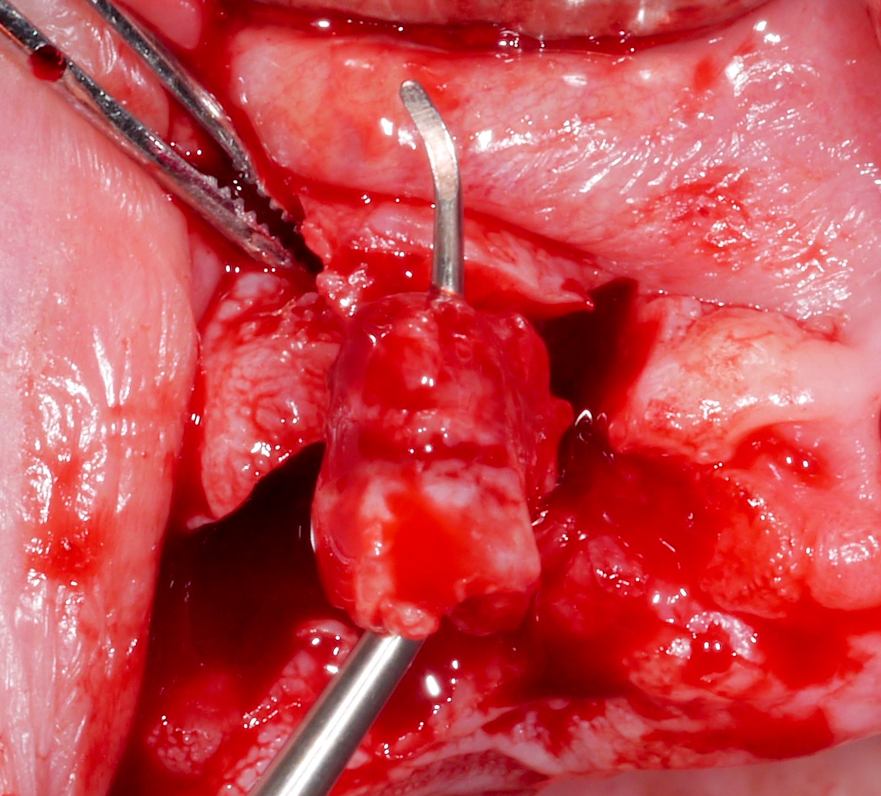
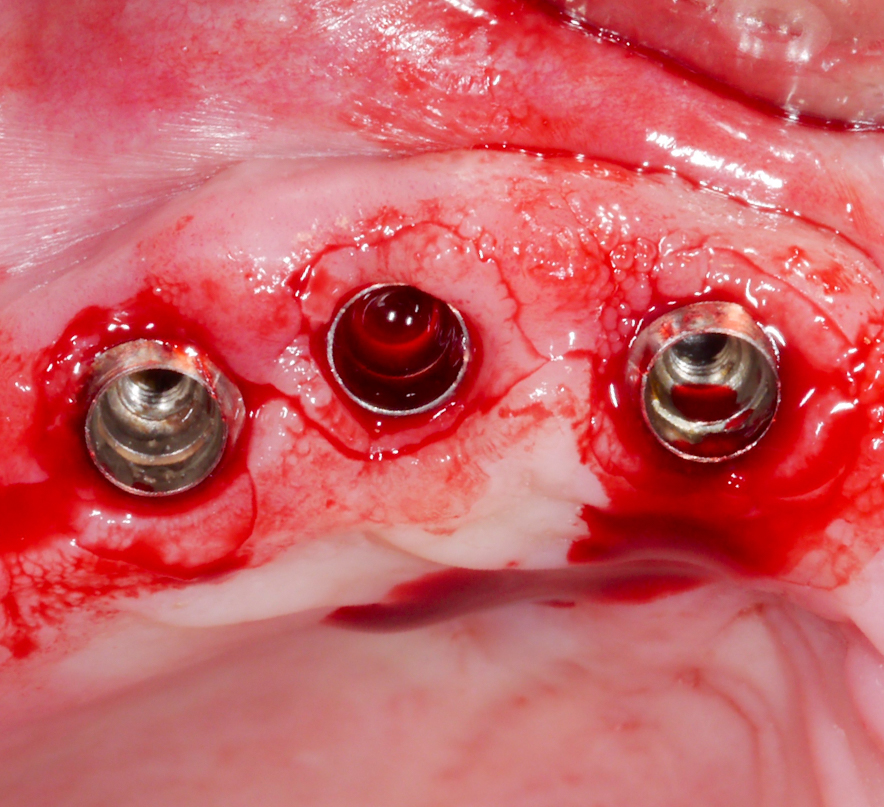
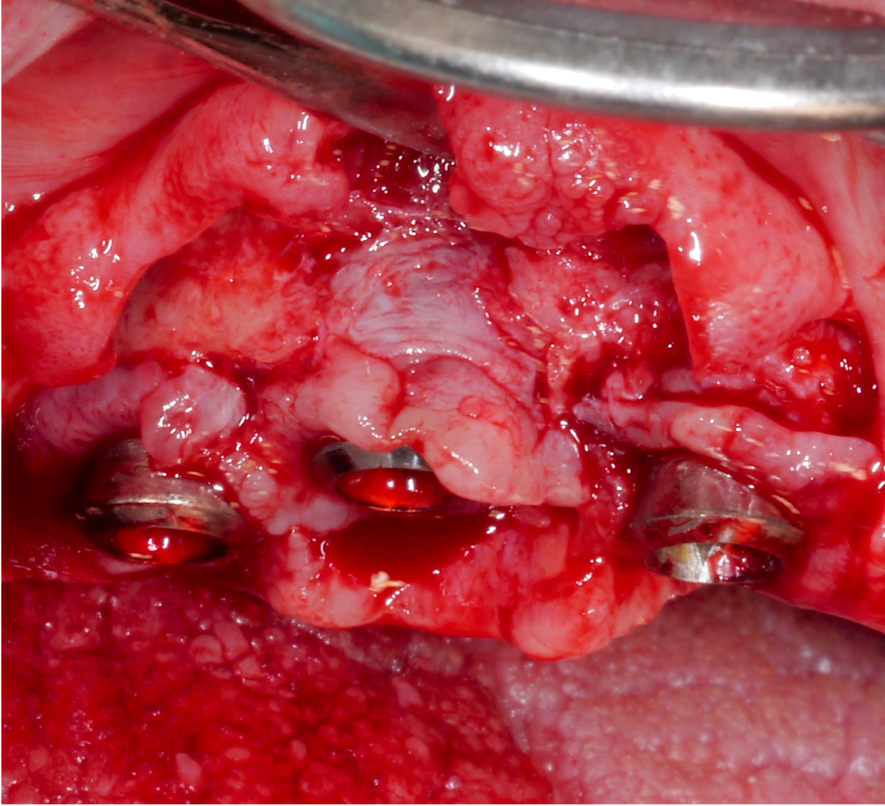
Approximately 3 – 4 weeks after the non-surgical treatment phase, the patient is scheduled for surgery. After raising the flap and removing the granulation tissue (Figs 4 and 5), the defect morphology is evaluated for the final treatment decision.
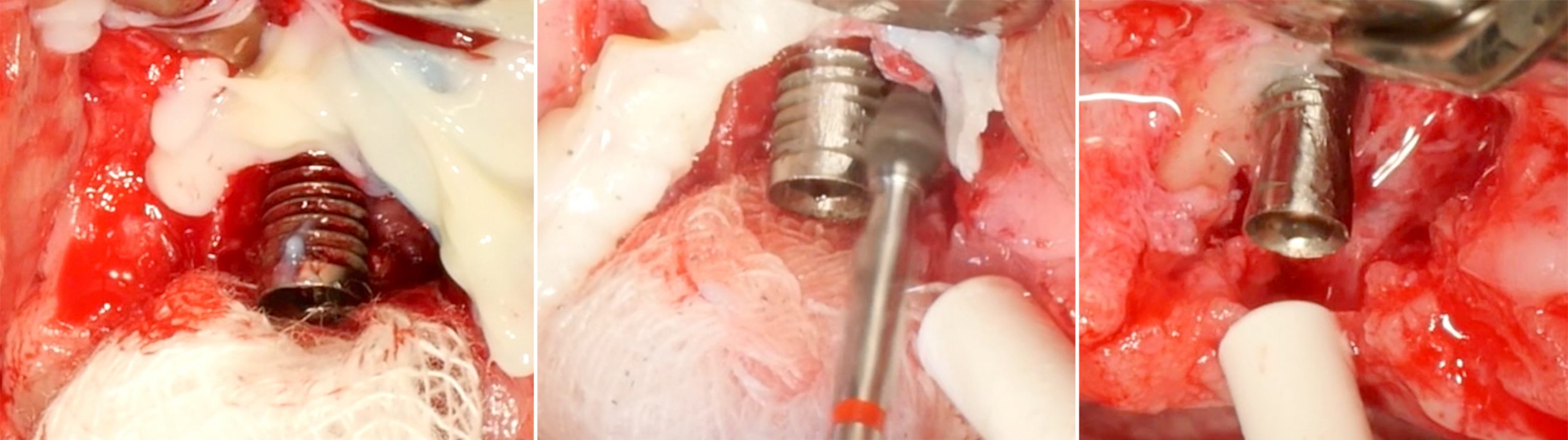
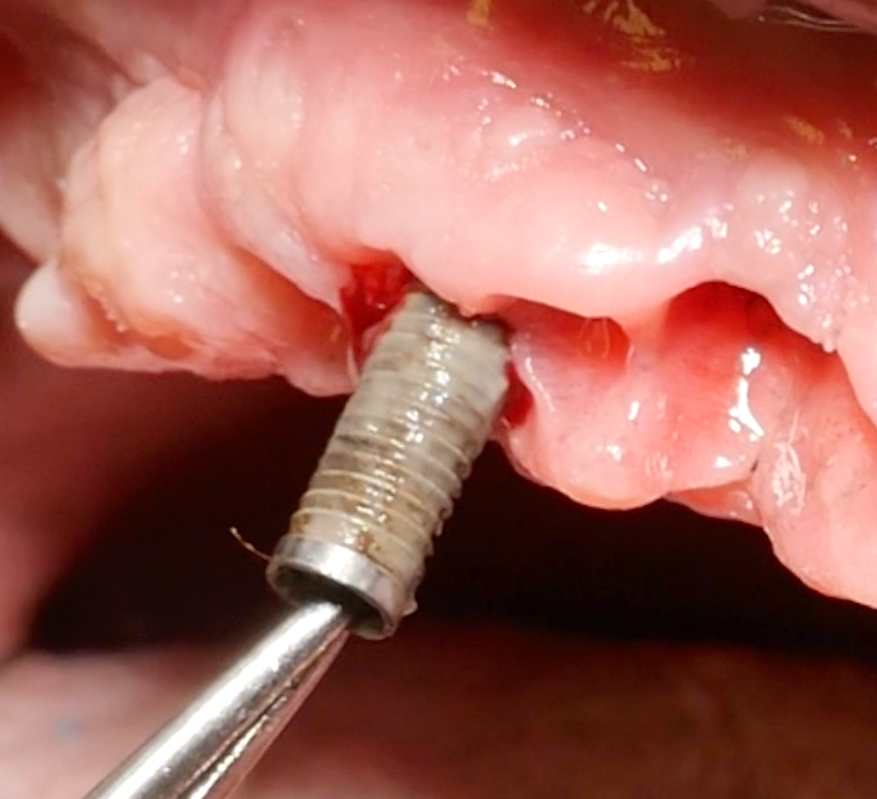
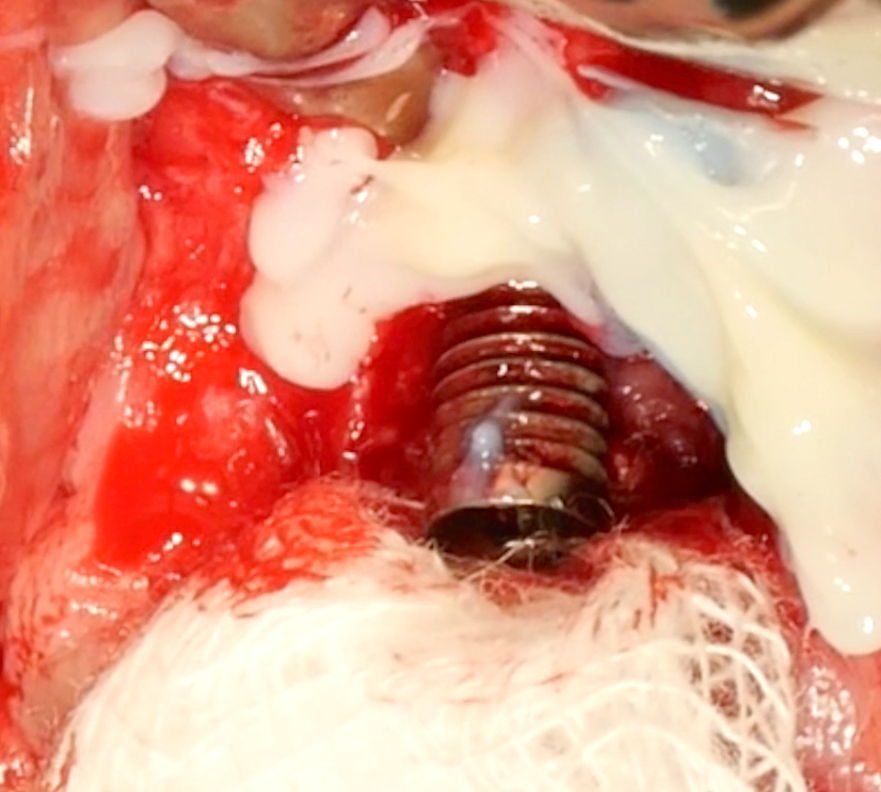
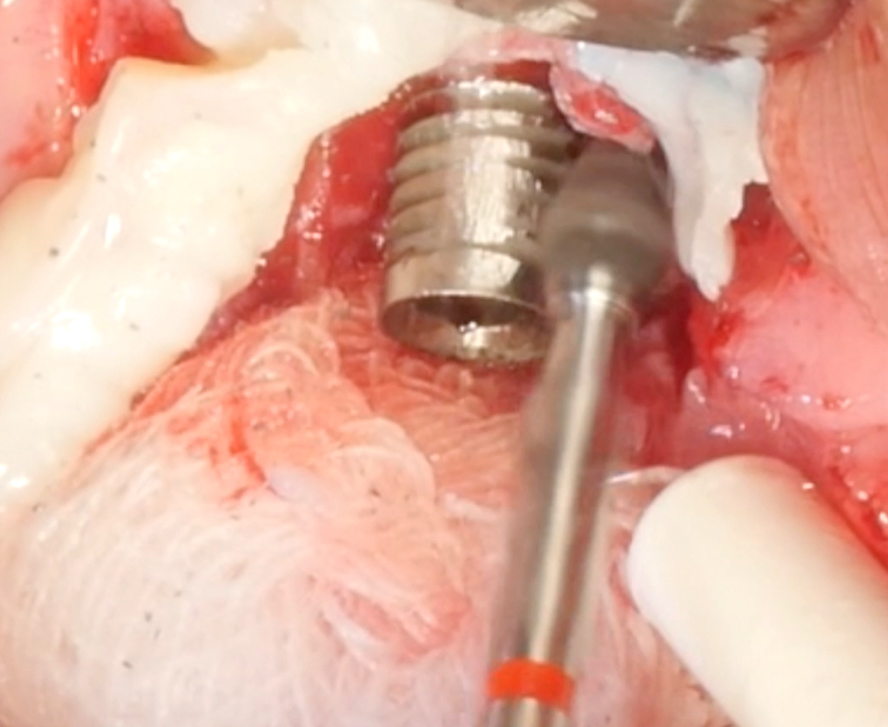
Implantoplasty should be performed under abundant irrigation to avoid overheating the surrounding bone (Sharonet al. 2013). Various methods have been proposed to remove the implant threads, such as diamond burs (Lozada et al. 1990), tungsten carbide burs (de Souza Júnioret al. 2016), and diamond sonic tips (Sivolellaet al. 2021), often followed by a polishing procedure with arkansas stones and/or silicone polishers. In a systematic review (Burgueño-Barriset al. 2021), the combination of tungsten carbide burs and silicone polishers was judged to result in the smoothest surface. However, based on the results of a recent in-vitro study, tungsten carbide burs (even without silicone polishers) appear sufficient to achieve a surface roughness similar to commercially available turned surfaces (Yildizet al. 2022). During implantoplasty the surrounding tissues should be protected as much as possible from titanium particle deposition (Fig. 6), and the shape of the bur is selected (Fig. 7) according to accessibility.
After the implant surface at the exposed aspect of the defect is smoothened with the appropriate sequence of burs (Fig. 8), the rest of the implant surface is disinfected.
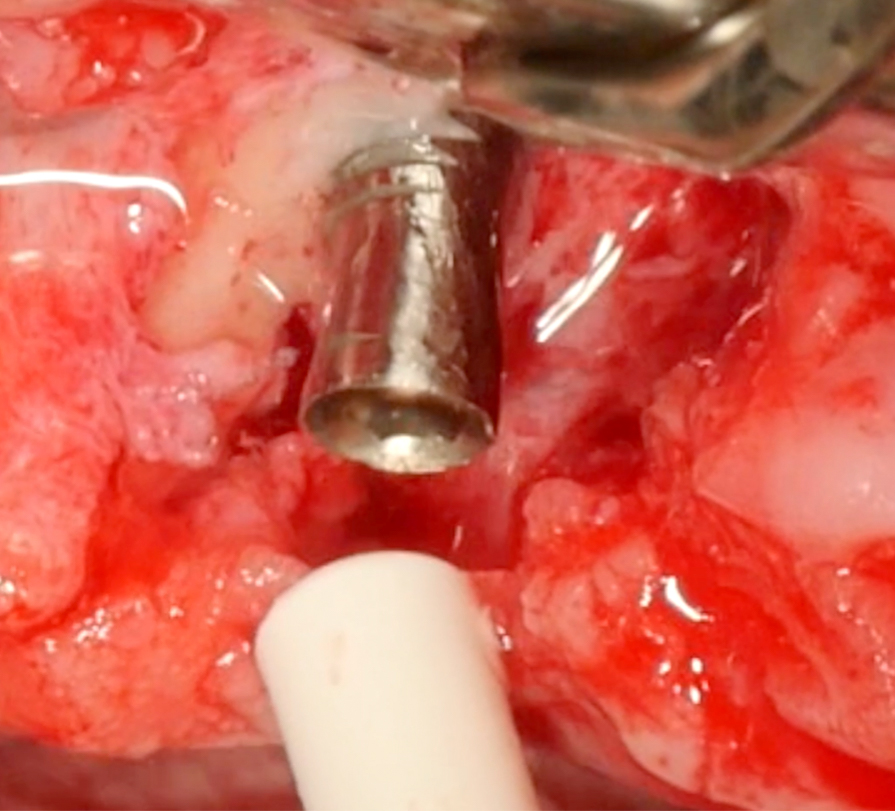
In this specific case, after implantoplasty, the implant was treated with 3% hydrogen peroxide. Any titanium particles deposited in the surrounding tissues are removed as far as possible. In resective approaches, this can be facilitated by performing the soft tissue resection from the internal aspect of the flap only after performing the implantoplasty.
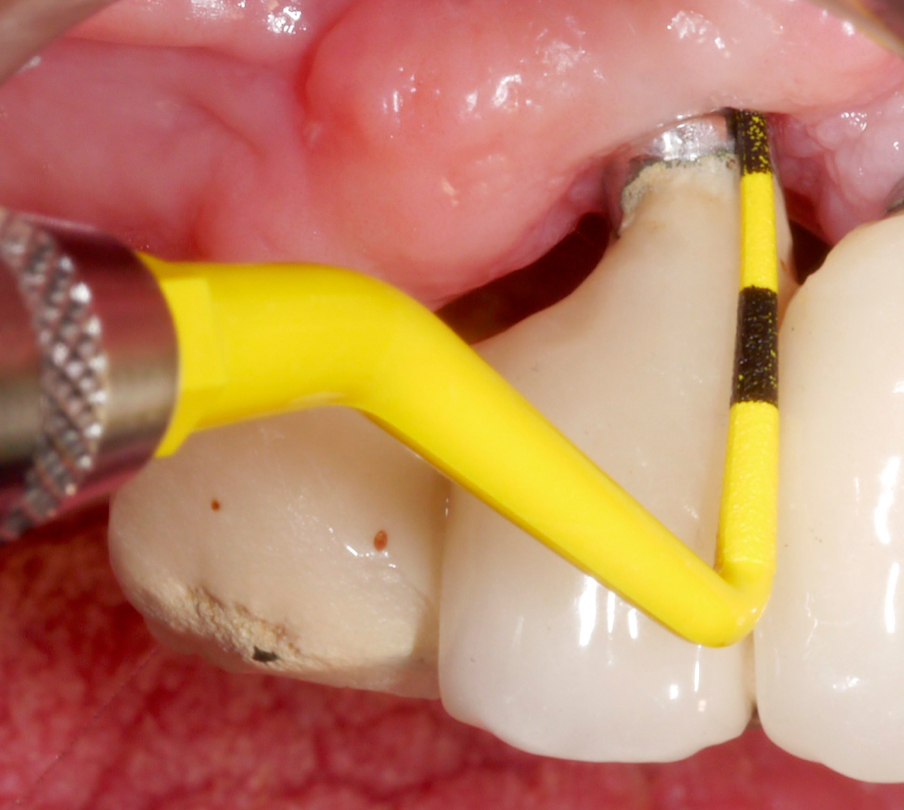
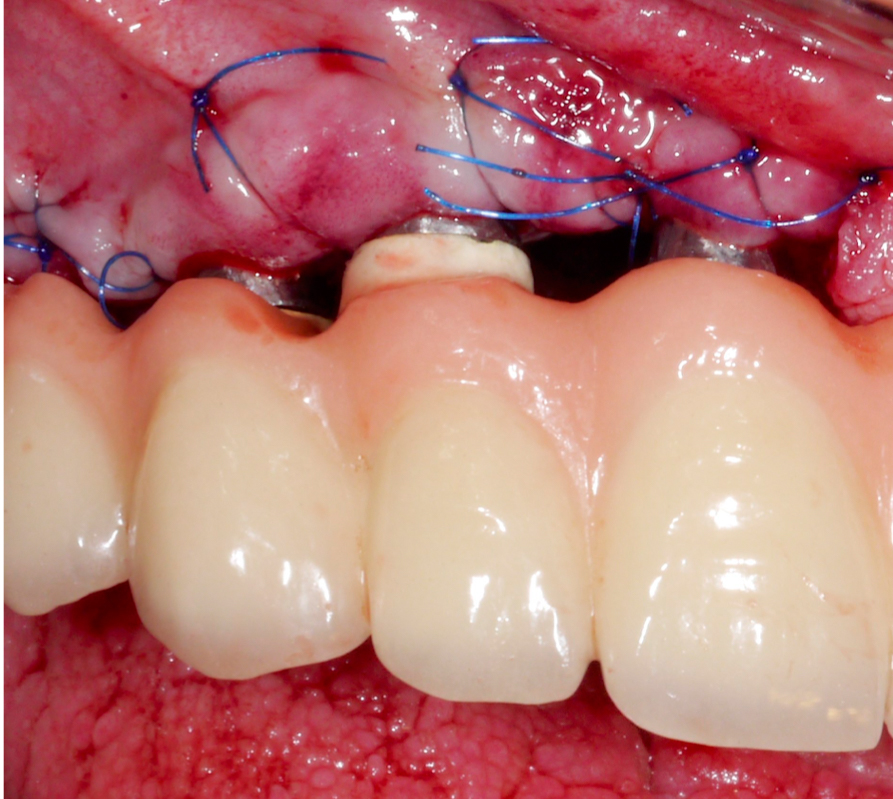
Once hard tissue re-contouring and soft-tissue trimming are completed, the flaps are sutured in an apical position to achieve sufficient pocket depth reduction (Fig. 9), and the prosthesis is then placed back.
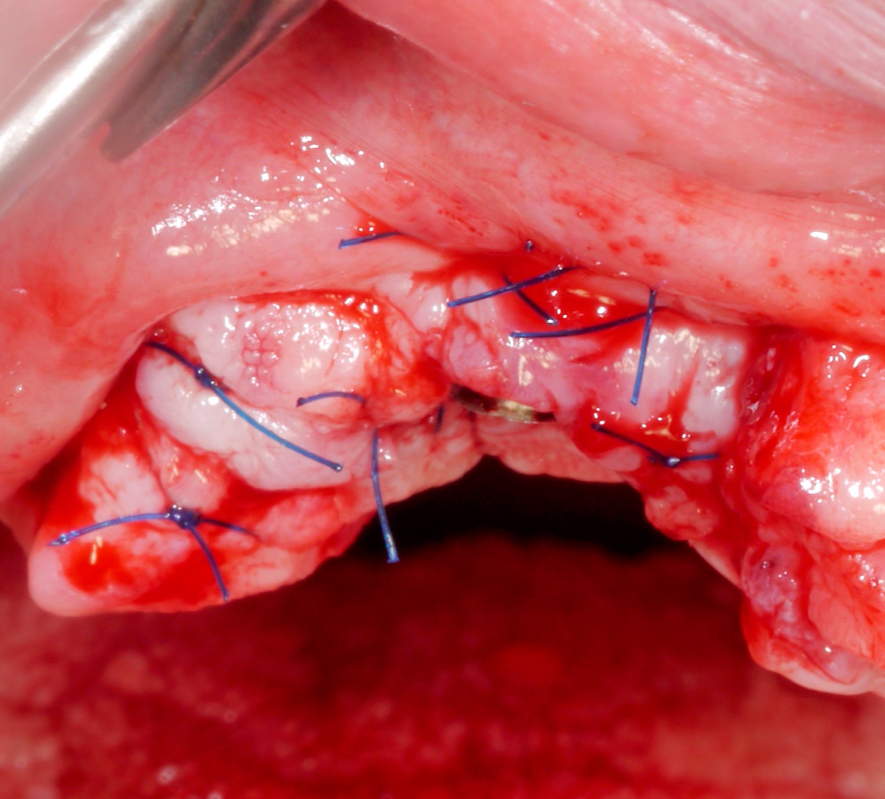
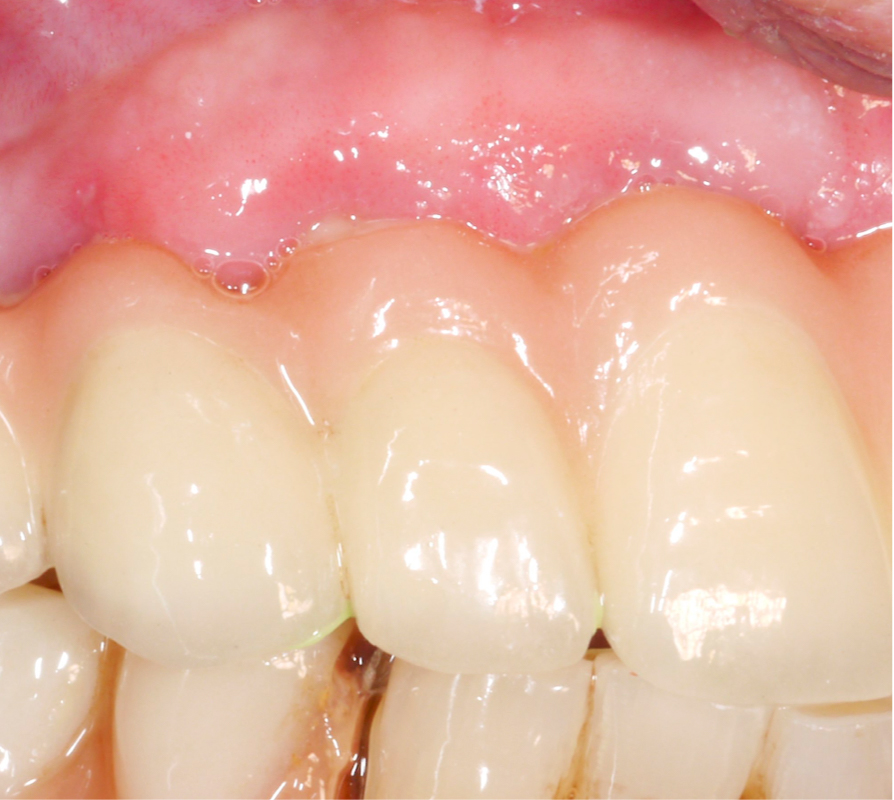
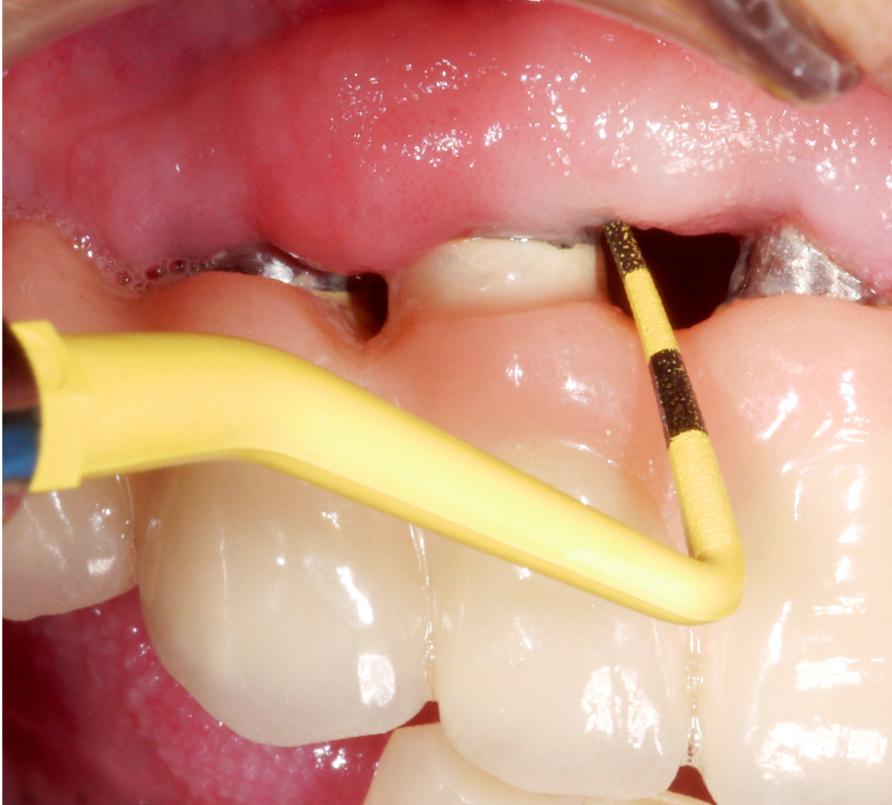
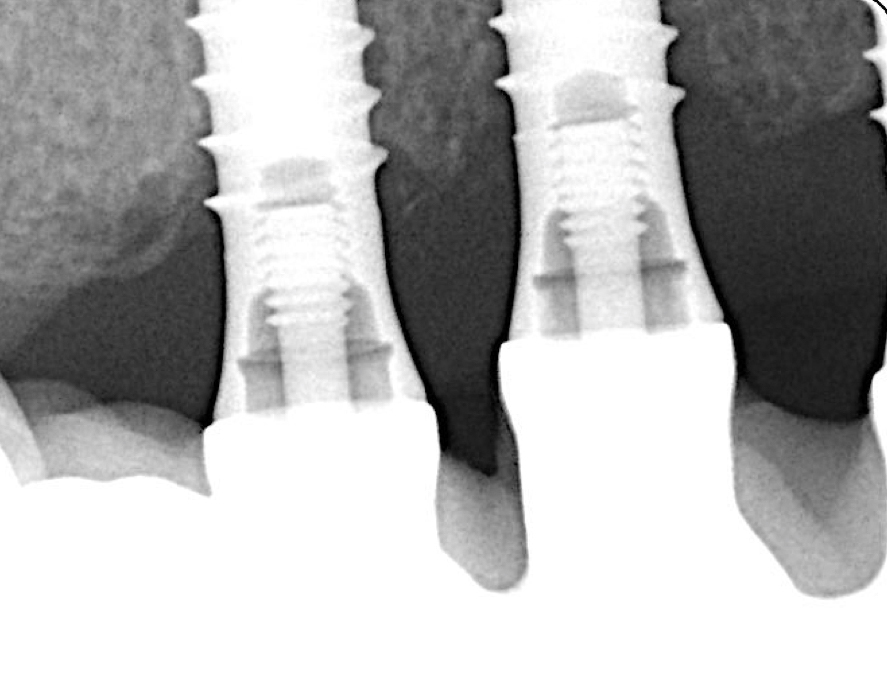
Three years after surgery the patient presented with stable peri-implant marginal bone levels and shallow pockets without signs of inflammation (Figs 10 – 11). The esthetic limitations of this procedure are obvious and have to be discussed with the patient in advance; in this case there was no issue due to a low smile line.
Case 2 – Combined surgical approach
Implants presenting with a more complex defect morphology often require a combined surgical treatment approach (Monje & Schwarz 2022; Schwarz et al. 2017; Schwarz et al. 2014). The present case involved a full-arch restoration on 6 implants, which were all affected by peri-implantitis. In the upper right, the central implant (in position #12) presented the deepest intra-osseous defect (Figs 12 – 14).
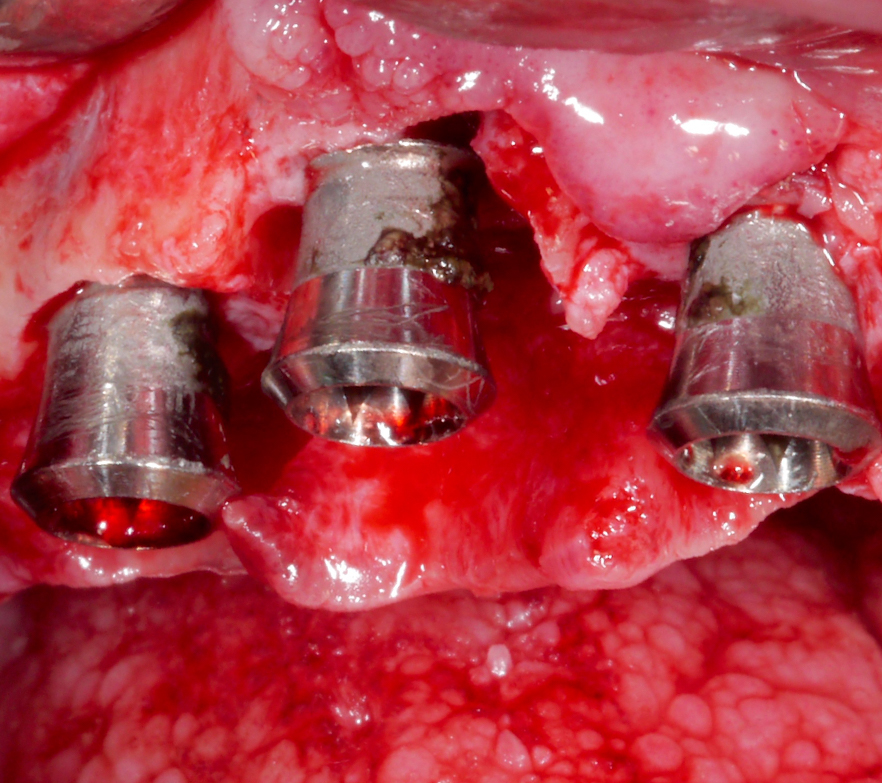
Following a thorough diagnostic evaluation and non-surgical therapy, a surgical approach aiming for reconstruction of the intra-osseous defects was planned. After raising the flap and removing the granulation tissue, the implant in position #11 presented with primarily horizontal bone loss, the implant in position #12 with buccal and palatal dehiscences and mesial and distal intra-osseous defects, and the implant in position #13 with horizontal bone loss and a mesial intra-osseous component (Figs 15 – 17).
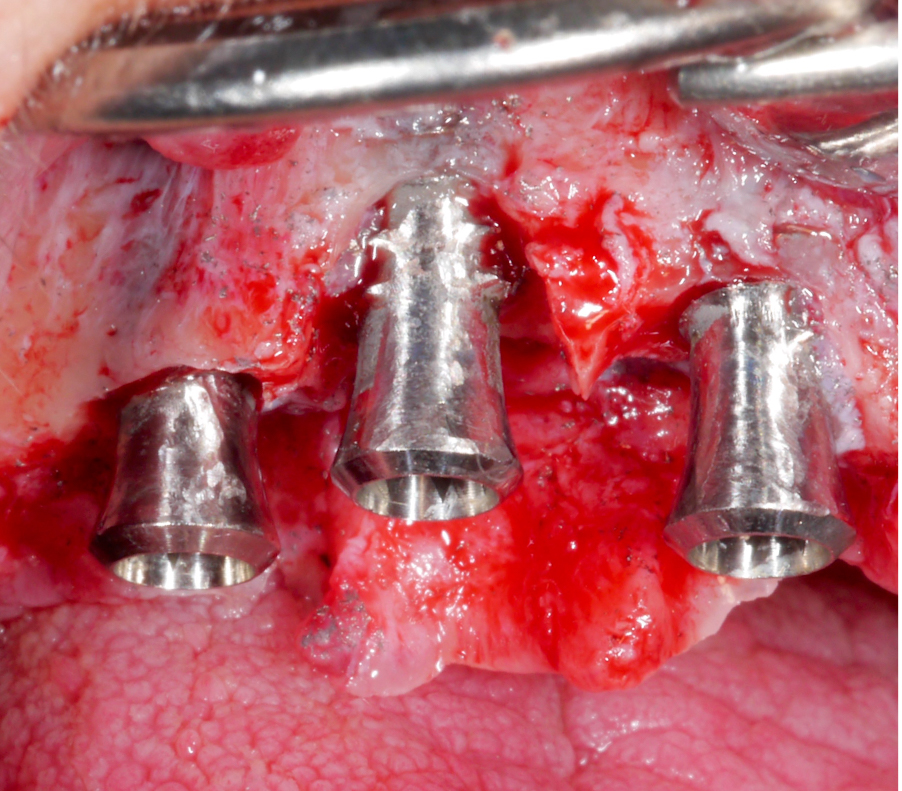
Corresponding to the defect configuration, a combined approach was chosen for the implants in position #12 and #13 and a resective approach for the implant in position #11; i.e., implantoplasty was performed on the supra-osseous components and at the buccal and palatal dehiscences, while the intra-osseous components were grafted with autologous bone chips (Figs 18 – 20).
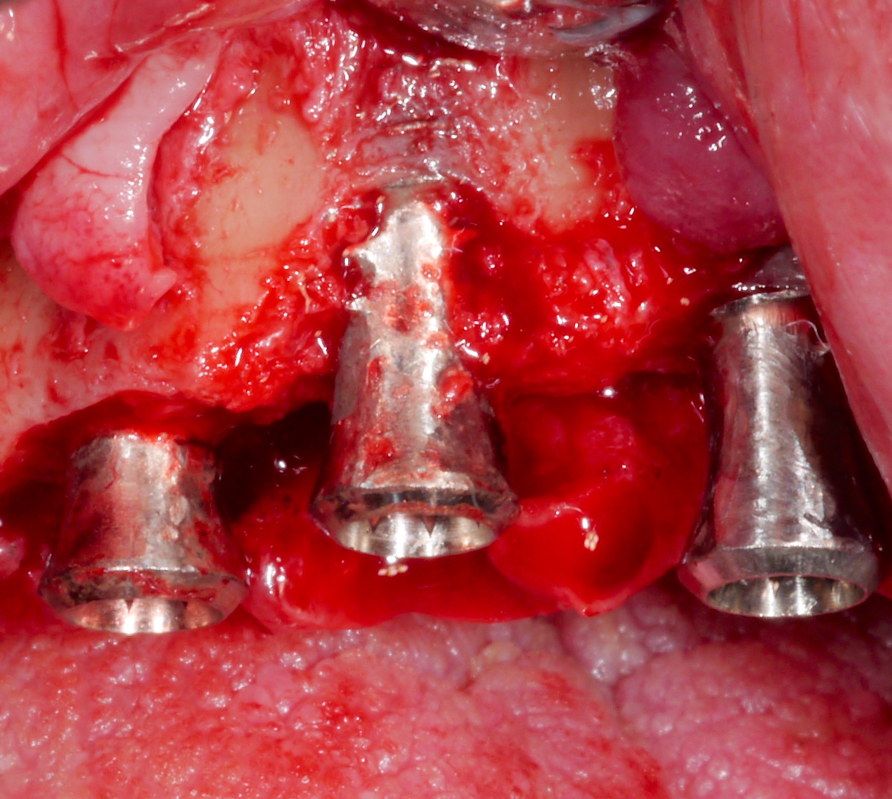
After performing implantoplasty and prior to grafting, the implant surface in the intra-osseous components should be decontaminated. Until now, no mechanical and/or chemical decontamination method has been judged as significantly superior (Baimaet al. 2022). In this case, debridement was performed with titanium curettes, followed by disinfection with air-polishing with erythritol powder, and subsequent 3% hydrogen peroxide application. The use of air-polishing devices has been reported to be superior in terms of biofilm removal in various laboratory studies (Steinet al. 2022; Iatrouet al. 2021; Sahrmannet al. 2015). For example, one of them (Stein et al. 2022) investigated the effect of various decontamination methods on a high-adherence biofilm consisting of 6 microbial species. Air-polishing with glycine or erythritol powder was proven effective in inactivating the biofilm independent of the implant surface (i.e., titanium or zirconium) without any evidence of cytotoxicity. Nevertheless, it should be noted here that intra-operative use of air-polishing is off-label.
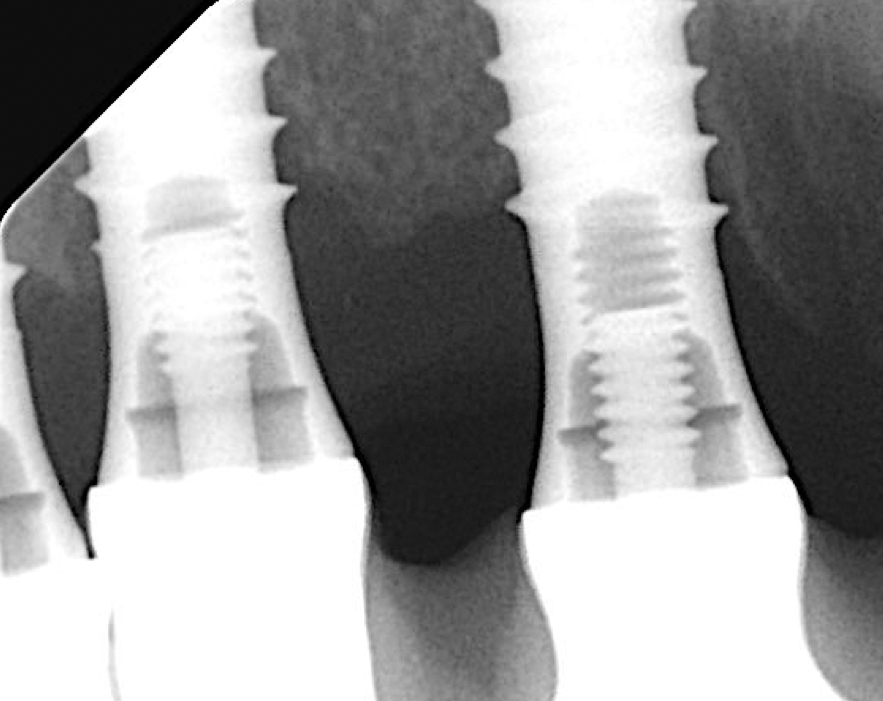
Two years post-operatively, the patient presented no clinical signs of inflammation, shallow probing pocket depths, and stable (implants in position #11 and #13) or improved marginal peri-implant bone levels (implant in position #12) (Figs 21 – 23).
Summary
Peri-implantitis treatment remains challenging in daily clinical practice and surgical intervention is often indicated. Occasionally, implantoplasty may be an option as an adjunct to surgery. The primary aim of implantoplasty is to obtain a smooth implant surface that allows better biofilm control by the patient. Clinical studies – although limited in number – have proven the efficacy of this procedure in achieving disease resolution and, until now, there are no remarkable complications reported.