Abstract
Various implant topographies are susceptible to peri-implantitis pathogen adherence and growth, resulting in biofilm formation. In combination with systemic, local, and implant-based risk factors, these pathogens can trigger an excessive inflammatory response in the peri-implant tissue, causing peri-implantitis onset along with hazardous bone resorption. Implant surface decontamination is an essential step in peri-implantitis therapy, aimed at completely removing causative bacteria from the implant surface without altering the implant structure. Once the inflammation has been treated, and disease progression has been halted, the additional treatment goal of peri-implantitis therapy is to promote tissue regeneration and/or repair with possible re-osseointegration. Various implant surface decontamination methods including mechanical, chemical, or physical methods have been proposed to prevent bacterial resistance development and surface damage. Photodynamic therapy (PDT) was introduced as a physico-chemical method of implant surface decontamination. PDT is suggested as an adjuvant method to peri-implantitis regenerative surgical therapy, resulting in successful implant surface decontamination following tissue repair and bone regeneration.
Introduction
In recent decades, modern implant dentistry, has grown rapidly and is facilitating the life of patients with partially or completely failing dentition. Numerous dental implant designs on the market aim to enhance effective osseointegration and achieve long-term success rates. Implant topography, emphasizing the implant surfaces, has gone through different modification processes in an attempt to accelerate and strengthen bone-to-implant contact rate and bone formation as well as to attain primary implant stability (Wennerberg & Albrektsson 2009). Unfortunately, with the launch of a large number of different implant macro-designs with different surfaces, implant complications have escalated quickly and now represent the greatest problem of the modern era in dentistry. The most common complication is biological and referred to as peri-implant disease, classified as peri-mucositis and peri-implantitis, the incidence of which increases yearly. In epidemiological studies, it has been estimated that the prevalence of peri-mucositis ranges from 19 to 65% while that of peri-implantitis ranges from 1 to 47% (Derks & Tomasi 2015).
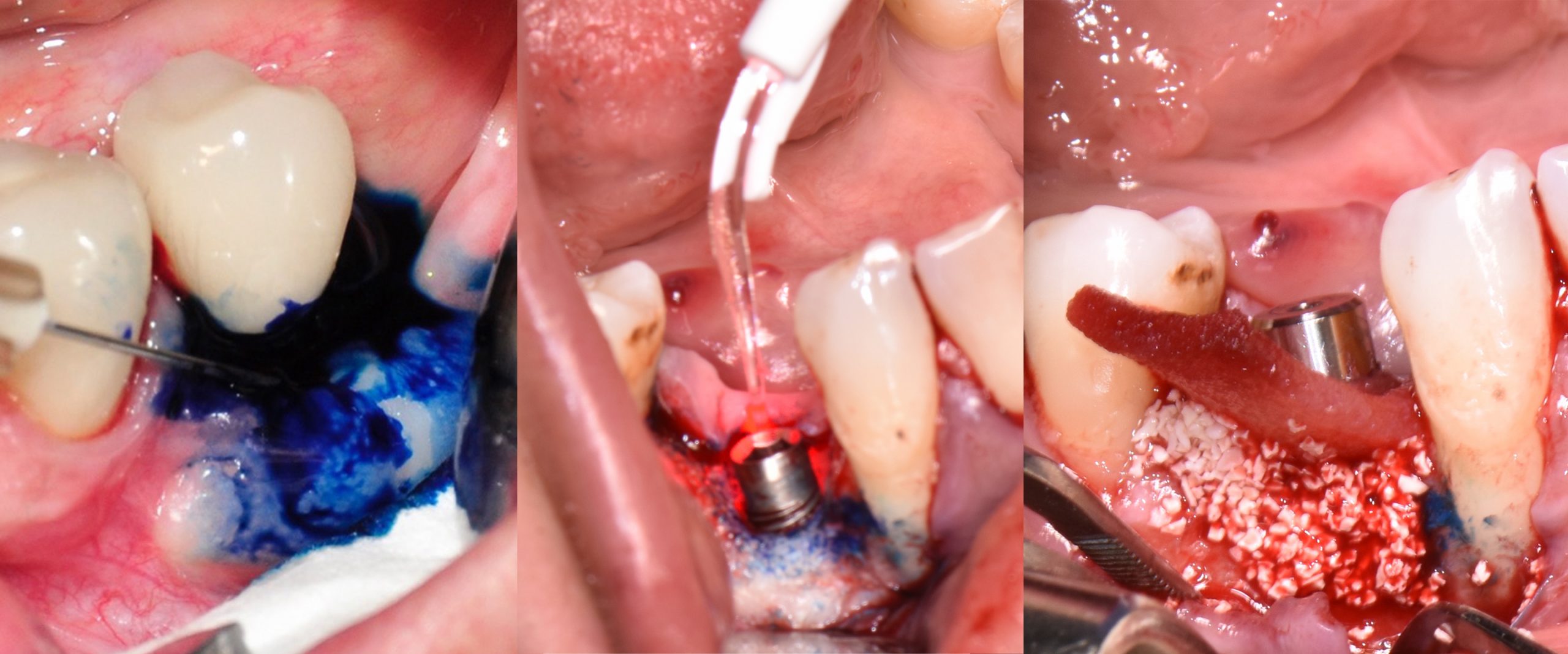
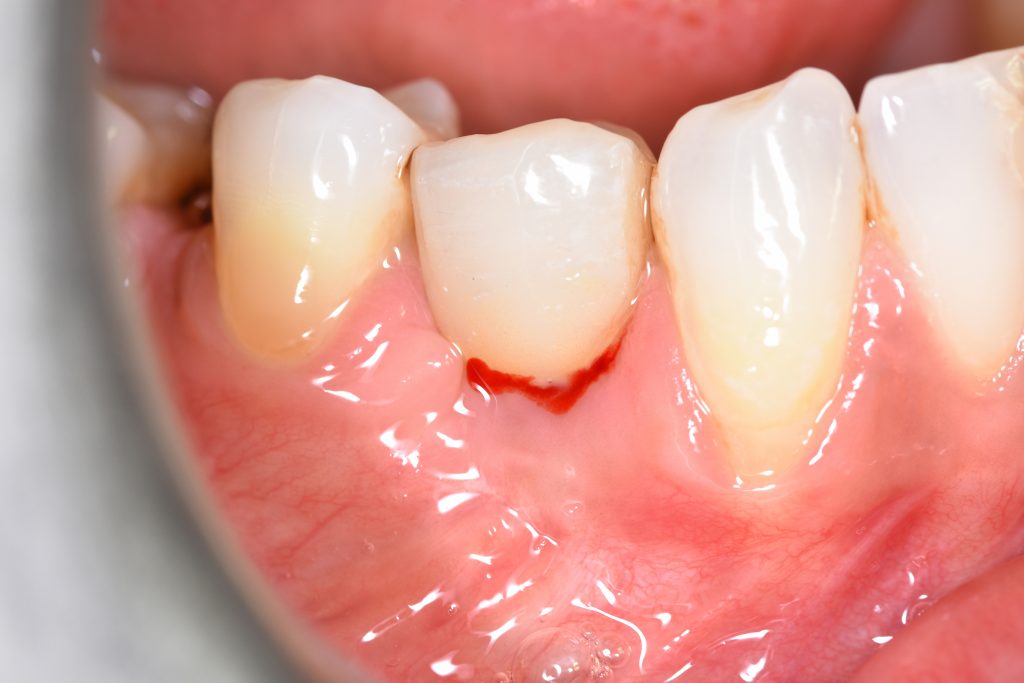
In the 2017, the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions (Berglundh et al. 2018), defined peri-mucositis as an inflammatory lesion affecting only soft mucosa characterized by bleeding on probing (BOP) and increased peri-implant pocket depth. In addition to the above-mentioned inflammatory signs, bone loss is indicative of peri-implantitis (Figs 1 – 2) (Sanz & Chapple 2012).
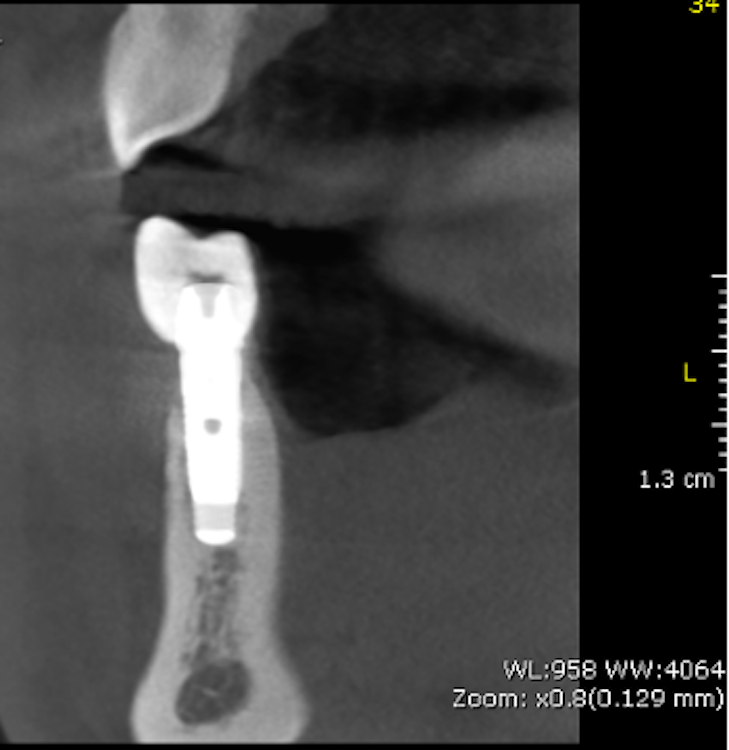
According to this classification, peri-implantitis is an inflammatory disease associated with a microbial challenge (Renvert et al. 2018) that may contribute to an immunological response in the surrounding tissue leading to marginal bone loss of more than 2 mm around an osseointegrated implant in function. In peri-implantitis, as a biofilm-associated infection, the presence of microbes is necessary but insufficient to cause the disease onset. Numerous systemic (i.e. genetic, diabetes mellitus, cardiovascular diseases, genetics, smoking), local (i.e. periodontitis, residual cement, poor oral hygiene), and implant-based and related factors (i.e. implant surface topography, implant placement localization and region, type of implant-supported prosthetic reconstruction, implant time in function) that stimulate and provide tissue breakdown are also involved (Derks et al 2016; Heitz-Mayfield et al. 2018; Rakasevic et al. 2018). Since both the etiology and the risks of the occurrence of peri-implantitis are known, appropriate disease management is one of the most important measures, in addition to prevention.
Implant surface influences peri-implantitis development
In the evolution of dental implants, different processes were undertaken in order to secure and enhance early osseointegration and minimize physiological bone loss in the first few years of implant loading (< 2 mm) (Suárez-López et al. 2016). Implant topographic features (material, coating, and microtopography), roughness, and hydrophilicity all play an essential role in the osseointegration process towards increasing bone-to-implant contact (BIC), reducing inflammation surrounding tissue, decreasing biofilm adhesion, and finally increasing primary stability (Teughels et al. 2016).
Implant material: Implant cores may be composed of titanium, ceramic, polymers, or be a hybrid type. Considering its extraordinary properties, including high strength, corrosion resistance, and fragility, titanium and its alloys are considered the material of choice in implant dentistry (Dohan Ehrenfest et al. 2010). Nevertheless, zirconia (ZrO2) ceramic implants are beginning to attract enormous interest due to their esthetics, allergy-free properties, soft tissue integration, and low affinity to plaque (Cionca et al. 2017). Zirconia implants also demonstrate less inflammatory response and marginal bone loss (Scarano et al. 2004; Liñares et al. 2016; Borges et al. 2020, suggesting they could be an effective solution to preventing peri-implantitis.
Implant surface modification and roughness: In the last century, the commonly used dental implant was based on machined surfaces, implying turning, milling, or polishing during the manufacturing process. For decades, novel modern technology was implemented to overcome the weaknesses of machined surfaces, resulting in numerous coating procedures to enhance implant features. Consequently, physical, chemical, and biological techniques were employed to modify the implant surface topography from machined (smooth) to rough. This led to distinctive surface roughness for dental implants – such as the minimally rough surface (0.5–1.0 μm), moderately rough surface (1.0–2.0 μm), and rough surface (> 2.0 μm) – and contributed to changes in implant physicochemical properties (Wennerberg & Albrektsson 2009). In clinical practice, implants commonly have moderately rough surfaces created through acid etching, sandblasting, anodization, and plasma spraying (Dohan Eherenfest et al. 2010). Furthermore, bioactive materials on the implant coating are added with the intention of not only enriching osseointegration but also stimulating bactericidal and/or bacteriostatic effects on the surrounding tissue to prevent biofilm formation (Ferraris & Spriano 2016). However, it is still unclear whether most modified surfaces can establish an anaerobic environment to produce different immunological reactions in the host tissue, changing from a stable to an active immune system (Albreksson et al. 2014). It was, however, found that an increase in surface roughness of > 0.2 μm influenced biofilm formation and bacteria adhesion (Teughels 2006). Various periodontopathogens, microbes, and fungi have been found to have an immense affinity to colonizing on titanium implant surfaces. Moreover, biofilms were reported to amplify the corrosion of the surface. It could therefore be assumed that implant roughness and implant surface coating could be contaminated, leading to the dispersion of micro- and nano-particles in the surrounding tissue that – together with corrosion – trigger the activation of an immunological response in the host tissue as a reaction to a foreign body, and consequently induce peri-implantitis and pathological bone loss (Wennerberg & Albraktsson 2009; Tughels et al 2006; Zitzmann & Berglundh 2008).
Implant surface decontamination strategy in peri-implantitis management
Peri-implantitis represents a huge problem in dentistry as it impairs implant stability and consequently causes implant failure. One of the major efforts in peri-implantitis treatment is to maintain long-term implant survival after treatment. The ideal treatment goal would be disease resolution with no further bone loss or inflammatory signs such as suppuration and bleeding on probing, together with healthy hard and soft peri-implant tissue (Heitz-Mayfield & Mombelli 2014). According to the 3rd ITI Consensus Conference (Proceedings of the Third ITI Consensus Conference 2003), the primary treatment goal for resolving peri-implantitis is to disrupt the biofilm and remove the calculus with particular attention to repositioning the margins of the restoration.
Various treatment protocols for peri-implantitis management have been proposed for non-surgical and surgical approaches combined with or without regenerative/resective procedures. However, non-surgical therapy achieves limited efficacy in the resolution of peri-implantitis, consequently leading to the recurrence of inflammation. Therefore, surgical therapy is advocated as a peri-implantitis treatment strategy.
Especially in regard to osseointegration and peri-implantitis, implant surface decontamination requires removing pathogens from the implant surface and maintaining the implant surface biocompatibility for possible re-osseointegration (Mombelli 2002). Anti-inflammatory treatment is suggested (Berglundh et al. 2018) to reduce soft tissue inflammation and stop disease progression along with various implant surface decontamination methods including mechanical, chemical, laser, photodynamic laser therapy, and implantoplasty. Many commonly utilized methods, unfortunately, demonstrated diminished effectiveness in removing pathogens when performed alone, with the additional risk of possibly damaging or altering implant surfaces. Surface damage could result in surface chemical oxide layer changes with the possibility of inducing corrosion and increasing surface roughness, thus promoting further biofilm re-growth on treated surfaces (Souza et al. 2021) and disease recurrence.
Current literature reports that disease cannot be resolved successfully by administering systemic antibiotics along with the application of chemical agents (i.e. chlorhexidine solution, saline) on moderately roughened implant surfaces during and after surgical peri-implantitis treatment (Carcuac et al. 2016). Furthermore, antibiotics should be administered with caution due to their well-known side effects and potential to contribute to the development of bacterial resistance (Mohsen 2020). Despite the many clinical and experimental studies conducted, an adequate protocol for surface decontamination has not yet been achieved due to insufficient evidence. Since using just one implant surface decontamination method provides an inadequate outcome, it should be mandatory to use adjuvant methods such as photodynamic laser therapy in conjunction with mechanical peri-implantitis treatment (Fig. 3).
What is photodynamic laser therapy?
Photodynamic laser therapy (PDT) was introduced as an alternative treatment modality to overcome antibiotic and antiseptic shortages. This method utilizes a laser device to activate a chemical component known as a photosensitizer (Fig. 4) with the goal of effectively eliminating pathogens. Contrary to a high-power laser, whose mechanism is based on energy production and increased temperatures (photothermal effect), the mechanism of PDT is based on a photochemical oxygen-dependent reaction (Rajesh et al. 2011) that involves three essential components: light, photoactive material (photosensitizer), and tissue oxygen. As it does not require a lot of energy and there is no increase in temperature during the treatment, PDT is considered a safe and painless method.
The working mechanism of PDT is based on the production of extremely reactive compounds, most notably superoxide radicals, such as single oxygen, which causes a toxic effect on the pathogen cell that ultimately leads to their destruction (Sharman et al. 1999). PDT has been demonstrated to be effective in treating various oral infections including periodontitis, delayed wound healing, endodontic root treatment with/without peri-apical lesions as well as treating oral lichen planus, herpes, and premalignant lesions and early tumors (Vohra et al. 2015). This suggests that PDT can also be effectively applied in the treatment of persistent and antibiotic-resistant strains (Garcez et al. 2010). The beneficial effects of PDT were demonstrated efficiently in the reduction of bacteria from various implant surfaces including machined, plasma-spray-coated, pure grade titanium alloy, zirconium, and anodized rough, Osseospeed, and MTX implant surfaces (Azizi et al. 2018; Gabric et al. 2020) without noting any surface damage (Marotti et al. 2013). Accordingly, PDT adjuvant therapy has been advocated for the peri-implantitis treatment strategy.
Clinical benefits of photodynamic laser therapy in peri-implantitis treatment
In clinical practice, photodynamic laser therapy is found to be effective in non-surgical (Bassetti et al. 2014) as well as surgical peri-implantitis therapy (Rakasevic & Gabric 2021). This method demonstrates a significant reduction in bacteria from various implant surfaces as well as being an effective alternative to local antibiotics. Considering the benefits of PDT, it should be considered as an anti-infective protocol for implant surface decontamination during the surgical therapy of peri-implantitis.
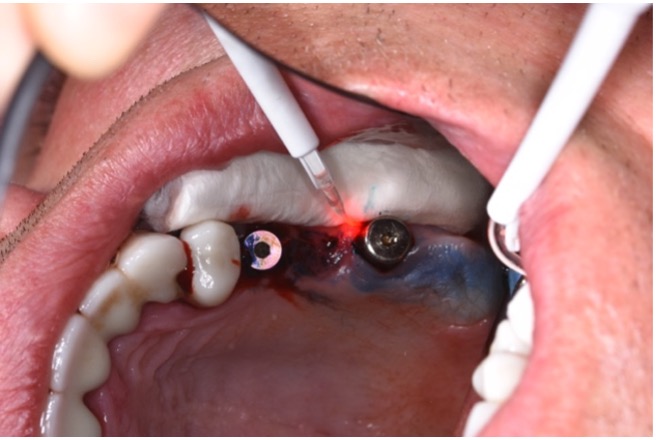
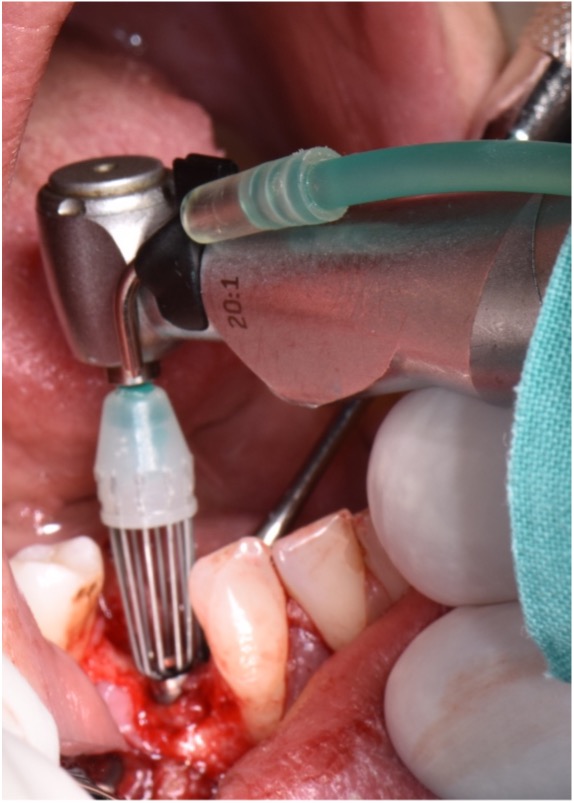
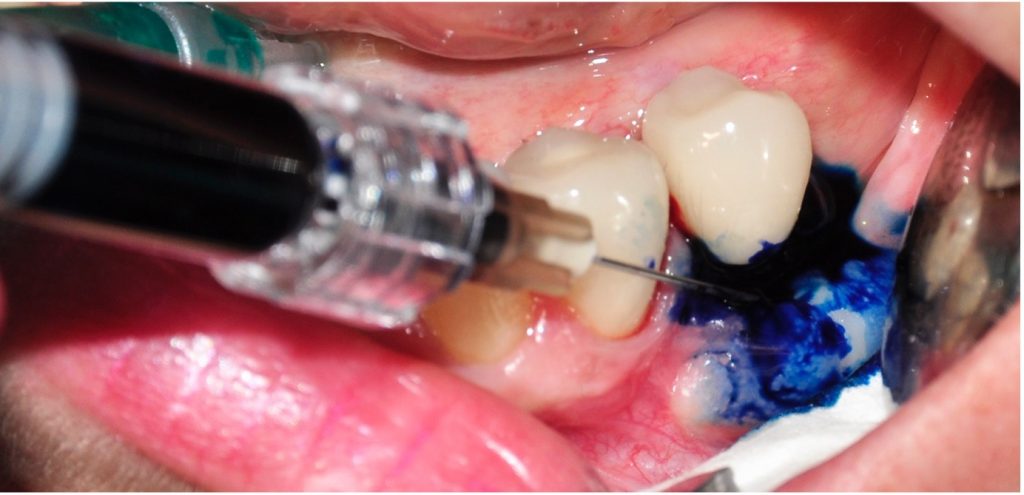
The first step in peri-implantitis management should be non-surgical therapy to reduce inflammation in the soft tissue. Two to four weeks afterward, surgical treatment followed by a full-thickness mucoperiosteal flap should be performed. The open-flap debridement allows complete removal of granulation tissue and more precise cleaning of the contaminated implant surfaces and surrounding tissue. Titanium curettes or titanium brushes should be utilized to remove granulation tissue and to decontaminate the implant surface followed by adjunctive PDT application. The photosensitizer should be applied on the implant surface and filled bone defect, thereafter, irrigated with saline solution and irradiated by low-level diode laser (etc. 660nm, 100mW) (Fig. 5).
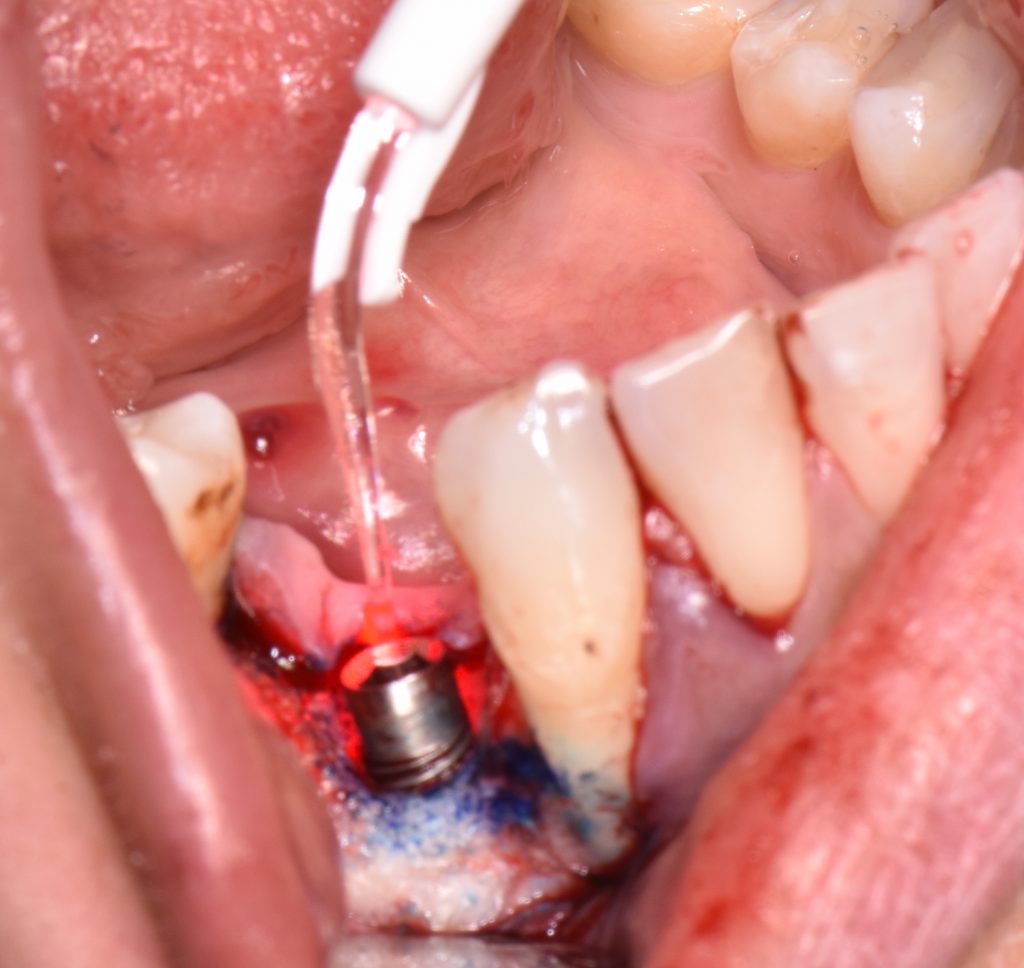
When aiming to achieve re-osseointegration, the intraosseous peri-implant lesion should be treated with a bone substitute/bioactive substance with or without a resorbable barrier membrane (Fig. 6).
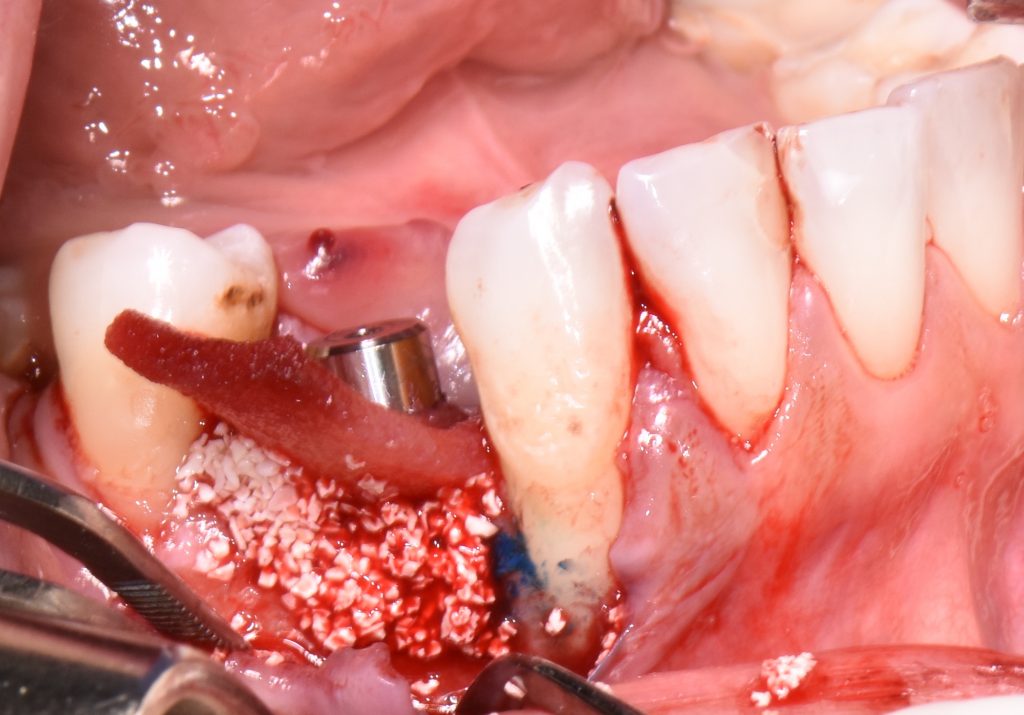
In postoperative care, knowing that PDT has antibacterial properties as well as being recommended as an alternative to antibiotic administration, systemic antibiotics could be skipped or reduced within three to five days after the above-mentioned procedure.
Short-term outcomes have demonstrated the positive influence of PDT on clinical and microbiological outcomes. Nevertheless, if PDT is combined with regenerative treatment approaches using bovine-bone xenograft with or without collagen membrane or dermal matrix, not only can the successful treatment of peri-implantitis be expected, but it also offers a great solution to achieve bone gain with possible re-osseointegration of implants with various surfaces including SLA, TPS, etc. (Dortbudak et al. 2001; Shibili et al. 2003; Rakašević et al. 2021).
Maintenance care
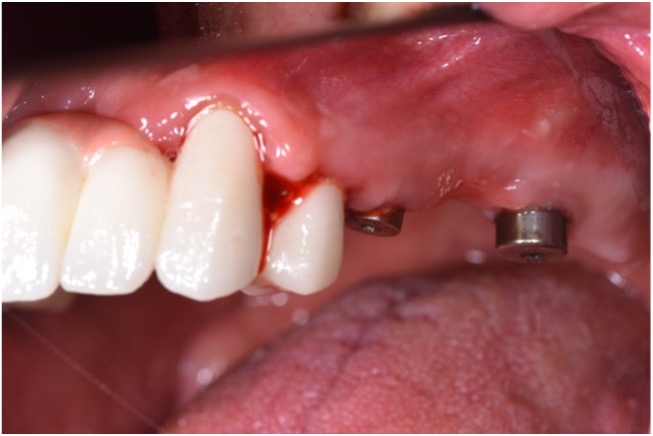
As peri-implantitis represents more than isolated events, treatment strategies should also address associated risk factors. Therefore, additional attention should be given to the possible resolution of peri-implantitis risk factors followed by proper supportive (maintenance) care. A high survival rate and a long-term successful outcome could be achieved if a supportive care program was involved in treating implants affected by peri-implantitis (Roccuzzo et al. 2018). Considering the greater success rate in eradicating pathogens and the potential for resolving peri-implantitis, PDT could also be recommended as one of the anti-inflammatory treatment modalities in daily practice that is followed by re-instruction and motivation on oral hygiene every three to six months as part of maintenance care (Figs 7 – 8).
Conclusion
The photochemical effects of PDT have been demonstrated to be beneficial against various bacteria, fungi, and viruses that cause oral infections. Moreover, this therapy is safe and has no side effects on the different implant surfaces, indicating that it should be considered as an additional strategy to mechanical treatments such as titanium curettes and titanium brushes. Lastly, with the improvement of clinical parameters as well as the possibility of re-osseointegration, photodynamic laser therapy might be considered as an adjuvant anti-infective protocol in peri-implantitis surgical treatment and supportive care.
Acknowledgement
My sincere thanks go to Professor Aleksa Markovic for his collaboration and contribution to the peri-implantitis projects, as well as for enhancing my clinical practice and research.