Introduction
This opinion article aims to, in part at least, assess the relevant literature on the concept of flapless versus flapped implant placement and offer a personal opinion on the conclusions that can be obtained. The long-standing traditional method of surgical implant placement is done by raising a full-thickness mucoperiosteal flap to visualize the underlying bone structure, as stated by Albrektsson et al. (1981). Once the implant is placed in the bone, either at the level of bone or soft tissue, the flap that has been raised is repositioned and sutured to enable primary intentional healing to begin. Raising a surgical flap has had many benefits for the operator, including visibility of the surgical site and ensuring that the implant is placed in the most appropriate position for prosthetic rehabilitation (Branemark et al. 1997).
The concept of the flapless implant placement technique was first developed in the 2000s, and several international authors claim to be pioneers. The use of this technique has continued to be very controversial. Different terminologies have been used to describe this technique, including ‘minimally invasive implantology’ and ‘atraumatic flapless implantology’, with some authors, even using the term ‘computer guided implantology’ to describe flapless implant placement. Since the flapless implant technique was first described, there have been different variations of the original technique involving a tissue punch, with some authors using small diameter drills to perforate the soft tissue instead of the traditional tissue punch. These developing adjunctive techniques further contribute to the various clashing opinions within the professional community.
Whilst the traditional approach to implant placement continues to be widely used, many clinicians are exploring flapless techniques and even employing a middle ground by using a flap-raising technique with a bespoke stent to guide implant placement during the surgical phase. These newer techniques, such as stent-guided and flapless implant placement, are more time-efficient and attractive options for patients as they are less surgically invasive. However, the more developed the techniques, the greater the armamentarium, resources, and costs involved (Jeong et al. 2009).
More generally speaking, dental implants are nowadays treatments of choice and treatments in high demand for their ability to rival natural esthetics as well as achieve optimal function for the patient. Implants also have high survival and success rates (Lemos et al. 2016). It must also be considered that the longevity of dental implants will depend on the influence of different primary factors associated with the implant surgery and secondary factors that may govern long-term marginal bone loss.
The surgical technique for the actual placement of the dental implant is one factor that has attacted a huge amount of controversy in the literature, with many conflicting opinions. There is now growing evidence that raising this full-thickness mucoperiostal flap temporarily disturbs and compromises the vascular supply to the soft tissue and bone. Therefore some evidence has emerged that this process leads to increased osteoclastic activity as well as crestal bone resorption and loss (Campello & Camara 2002).
There is also growing biological plausibility that in avoiding the need for flap creation and reflection, implant surgery can be done through the gingival tissues by flapless means, without the need for flap creation and retraction and that this protocol allows the gingival tissues to maintain an intact vascular supply, therefore leading to less crestal bone resorption and loss (Jeong et al. 2009).
Learning from the Literature
The study by Sunitha & Sapthagiri (2013) examined 40 patients undertaking implant surgery, half the group undertook flapless techniques for implant instalment and the other half had traditional flapped instalments where a full thickness flap was raised. The crestal bone levels at instalment and 2 years after implant placement were measured and recorded on peri-apical radiographs. The study concluded that flapped surgery lead to significantly higher bone loss 2 years after implant placement than flapless surgery. Results in line with this conclusion have been demonstrated by the study performed by Job et al. (2008) which concluded that when the changes in crestal bone height with implants placed flaplessly and flapped were compared, the flapless implant approach showed statistically significant lesser reduction in crestal bone height.
Tsoukaki et al. (2013) performed a study, which further agreed with the conclusions drawn by the previous studies. The study concluded that the peri-implant sulcus depths were significantly higher in the flapped implant group at both 6 and 12 postsurgical weeks. Flapped implants showed crestal bone loss, whereas no bone resorption was detected around flapless implants.
However, opposing results have been put forward by other studies, including those elegantly performed by Froum et al. (2011) and De Bruyn et al. (2011). Both these studies stated that there were no significant differences between tissue or bone outcomes when comparing the results of flapless and flapped surgical implant techniques.
Froum et al. (2011) demonstrated that there were similar levels of probing depth in flapped and flapless groups as well as similar rates of BOP (22.8% vs 17.9%, respectively). Papilla levels increased during the first year in both groups. De Bruyn et al. (2011) demonstrated that both techniques, flapped and flapless, resulted in increasing bone loss during the first year. It must be noted that there was a higher degree of bone loss in the flapless group than for sites where implants were placed using the flapped technique. After this initial healing timeframe, no further bone resorption was observed and bone loss in both groups was statistically equal.
In summary, there are numerous protocols that may be employed for the placement of dental implants, each with a different level of invasiveness. It is also plausible that the risks involved with implants that are flaplessly placed are more to do with a surgeon’s experience and skill. It is likely for further risks to emerge when implants are placed flaplessly using the freehand technique. However, with the advent of 3D imaging and cone-beam computed tomography (CBCT) being more widely used for implant surgery, it is likely that implants will be able to be placed and restored with the most optimal prosthetic outcome in mind, and as such surgical guides will improve the accuracy and success rates of implant surgery, regardless of which surgical technique is employed (Lemos et al. 2016).
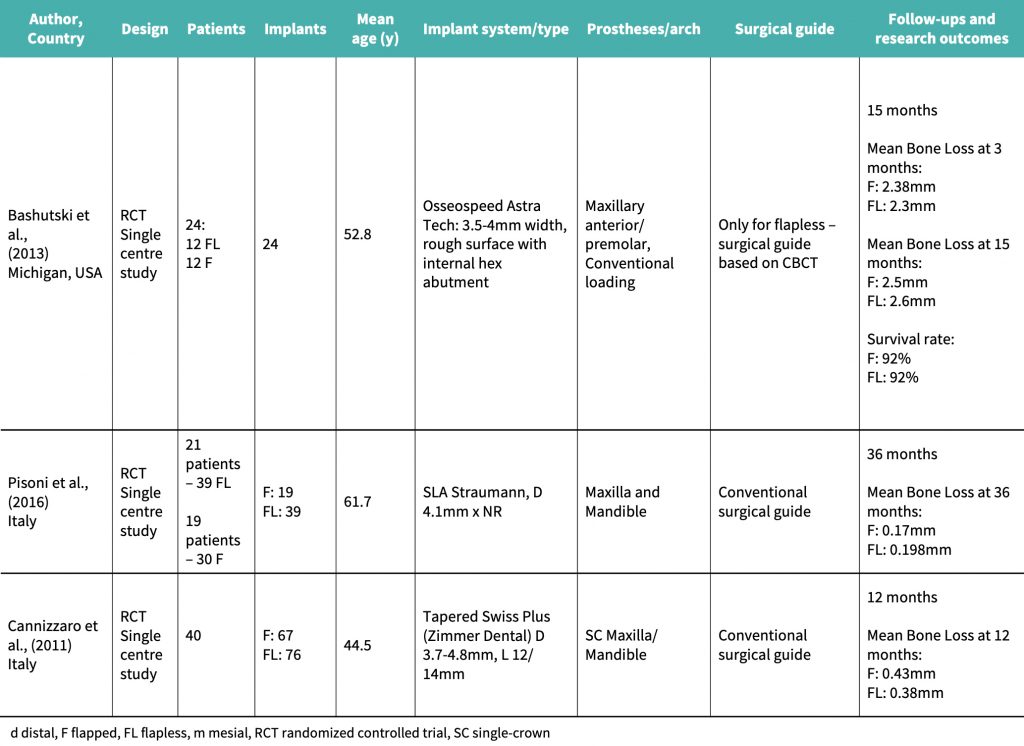
Bashutski et al., (2013) performed a single-center randomized controlled trial (RCT) with a sample size of 24 patients, each receiving a single anterior maxillary implant. 12 patients undertook flapless surgery, and 12 underwent traditional flapped surgery for implant placement. Each potential implant site had to have a minimum 6.5-mm bone thickness and 10-mm bone height, and 2 mm of keratinized mucosa. Two periodontists performed all surgeries, and one other examiner measured bone level changes over time on periapical radiographs.
The major weakness was the very small sample size of 24 patients. Also, during flapless implant placement, implants were placed at the level of crestal bone, whereas with flapped surgery, the implant was placed subcrestally, which could have clinical implications influencing the pattern of results. The study also did not document the starting point of the bony anatomy before implant placement took place, for example, the dimension or presence/absence of buccal ridges. This pre-operative anatomy would influence healing as well as final hard and soft tissue outcomes and so should have been documented.
The strengths of the study are that a variety of factors were measured, including papillary index, plaque, tissue levels, biotype, and keratinized mucosa changes. The implant was standardized, as all implants were placed in maxillary anterior or premolar teeth. Follow-ups were done at 3 days and thereafter at 3, 6, 9, and 15 months with regular long cone periapical radiographs (LCPAs) being taken with custom-made stents and holders to standardize the radiographic technique and positioning. CBCT examinations were performed pre-operatively and at 15 months postoperatively. Another strength was that one periodontist measured the bone levels at the end of the study, and two periodontists performed all the procedures. The examiners were blinded, which minimized any bias over results. All implants were micro-threaded, platform-switched, and had a fluoride-modified nanostructure. The loading protocol employed for the study was conventional and standardized for both flapped and flapless groups, and loading was done by way of definitive crown placement 3 months post-operatively.
Pisoni et al., (2016) performed an RCT in Italy, where 40 patients were treated with 69 implants. 21 patients were treated with 39 flapless implants and 19 patients with 30 flapped implants. There was no statistical difference in the location of implant surgery except that maxillary implants were only used in 6 cases for the flapless group and in 3 cases for the flapped group. Crestal bone-to-implant distance was measured pre- and post-operatively by periapical radiography using radiographic stents to ensure correct angulation and accurate bone loss measures. All implants were loaded at 3 months in the maxilla and 2 months in the mandible.
This study relied mainly on radiology and probing depth to indicate bone loss. However, probing depth is a surrogate measure of bone loss, and bone sounding should have taken place instead to allow for accurate bone level data. Combining bone-sounding and radiographic measurements would give a more accurate representation of actual bone loss. CBCT imaging should have ideally been employed as well, as this gives a more accurate overview of bony changes over time. With relatively low CBCT dosage this offers an ideal method of bone measurement. The use of the same implant, surgeon, and loading protocol, whether implants were placed via flapped or flapless means, is advantageous as it allows for effective comparison between cohorts during the long-term follow-up. The study does have further merits in that there was randomization of the patients undertaking the type of surgery in the form of coin flip. One surgeon performed all surgery, and stents were used for both cohorts to give both groups an equal chance of prosthetic excellence in placement position and angulation.
Cannizzaro et al., (2011) performed a split-mouth RCT with 40 patients, each with 2 separate edentulous regions, with a minimum 5-mm thick bone, 10-mm height, and at least 1 potential implant site on each side of the mouth. Tapered transmucosal implants were placed. Marginal crestal bone was measured via LCPA radiology at the time of implant placement, and 12 months post-operatively. In total, 76 implants were placed flapless (25 of which were post-extraction immediate placements), and 67 implants were placed flapped (24 of which were post-extraction immediate placements). Patients undertaking the implant surgery were allocated into 3 groups – non-smokers, moderate (less than 10 cig/day) smokers and heavy smokers (more than 10 cig/day). Cannizzaro, an experienced surgeon, performed all implant surgeries and also prosthetics. All definitive crowns were placed after 2 months, and, in the short term after surgical placement, any implant achieving a torque of 48 Ncm, was immediately loaded with an acrylic crown. Patients were randomized for the surgical procedure. Additionally, the assessors reviewing the radiographs and also obtaining clinical measurements were blinded to the surgery type that had been performed.
Overall, the study’s main weaknesses are the very small sample size and the minimal time frame under investigation, as well as the fact that there is no consistency in anatomical location, as some implants were placed in the maxilla and others in the mandible. There is also no consistency in the method of implant installments, as some were done as immediate placements, others were done in a stable bony platform as well as the fact that some implants were immediately loaded. In addition, we are unaware as to how many assessors reviewed clinical parameters and the radiographs, and therefore each individual assessment technique could have resulted in influence over the data. Smoking was not fully accounted for, although smokers were labeled with significance of their habits for result assessment. However, the many strengths of the study outweigh these weaknesses. Firstly, the split mouth nature allows for patient variables to be controlled, and the grouping into 3 additional subcategories, allows smoking to be, somewhat, controlled as well, in contrast to many other studies which include smokers who are deemed mild (less than 10 cig/day). In addition, having one surgeon perform all the work has additional merits, as there is no variation in surgical or restorative technique allowing for direct comparison. The fact that the same implant system and design were used as well as the same definitive loading protocol (after 2 months, and only if 48 Ncm torque was achieved was the implant loaded immediately with a temporary crown), has additional merits, as well as the fact that grafting was not used in the study procedures, providing a true representation of the surgical technique impact on the patient’s biology. OPG, LCPA, and bone calipers were all utilized in the information-gathering process, allowing for the analysis of multiple parameters of hard tissue loss and therefore providing a very accurate result of bone loss. Randomization via the envelope technique after the patient had been anesthetized was an effective concept. Outcome assessors were blinded, and the situation reflected real clinical conditions.
Results
A summary of the results obtained can be seen in the results table:
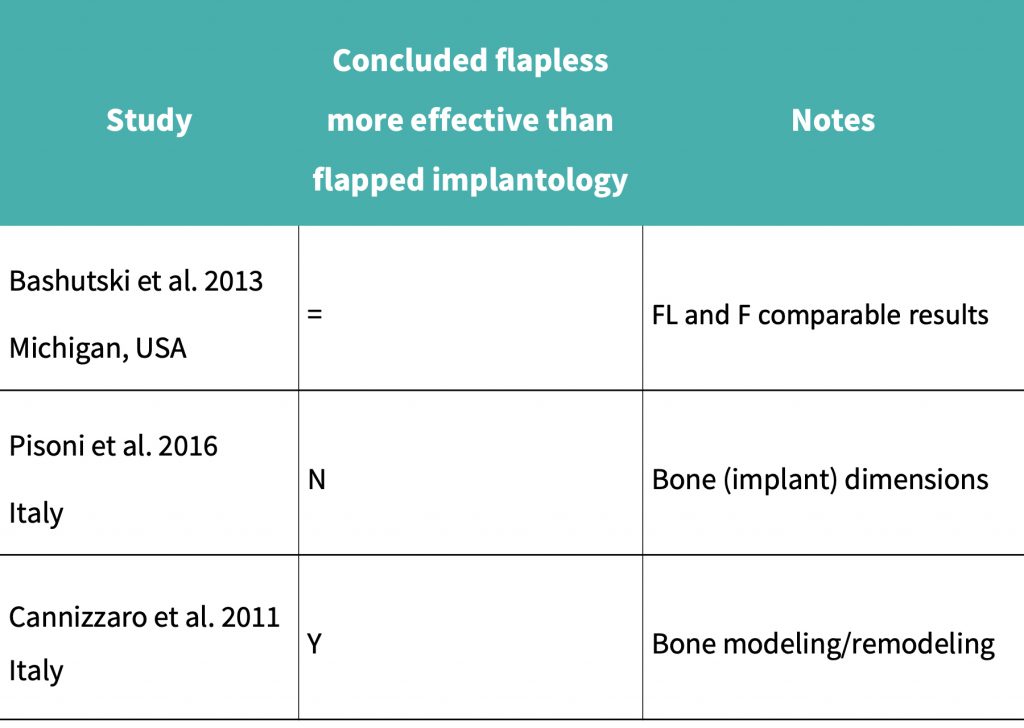
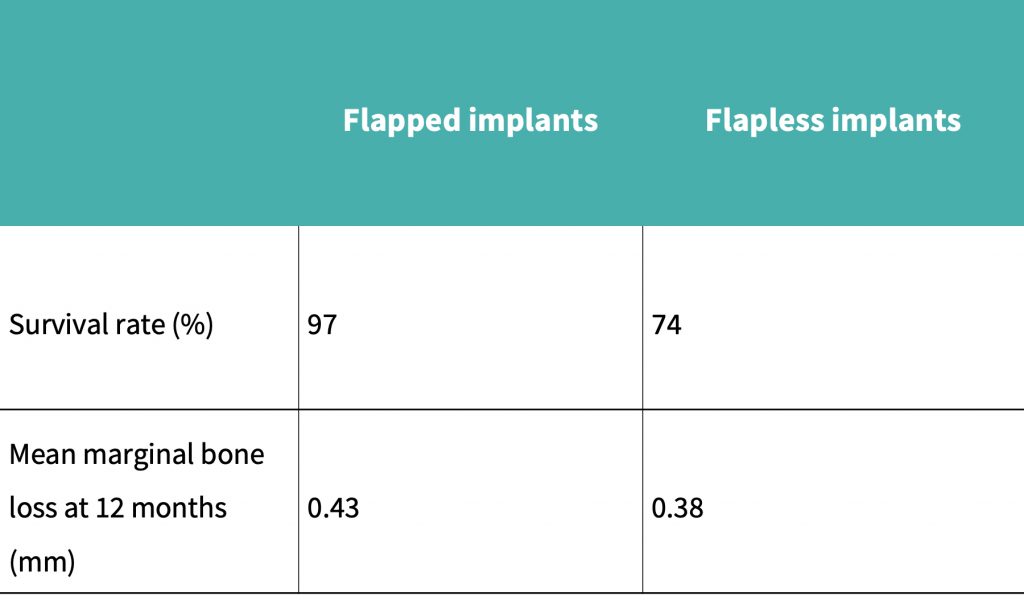
Cannizzaro et al. (2011) performed a split mouth RCT over 12 months which concluded that mean bone loss after 12 months for the flapped implant cohort was 0.43 mm, and for the flapless implant cohort it was 0.38 mm.
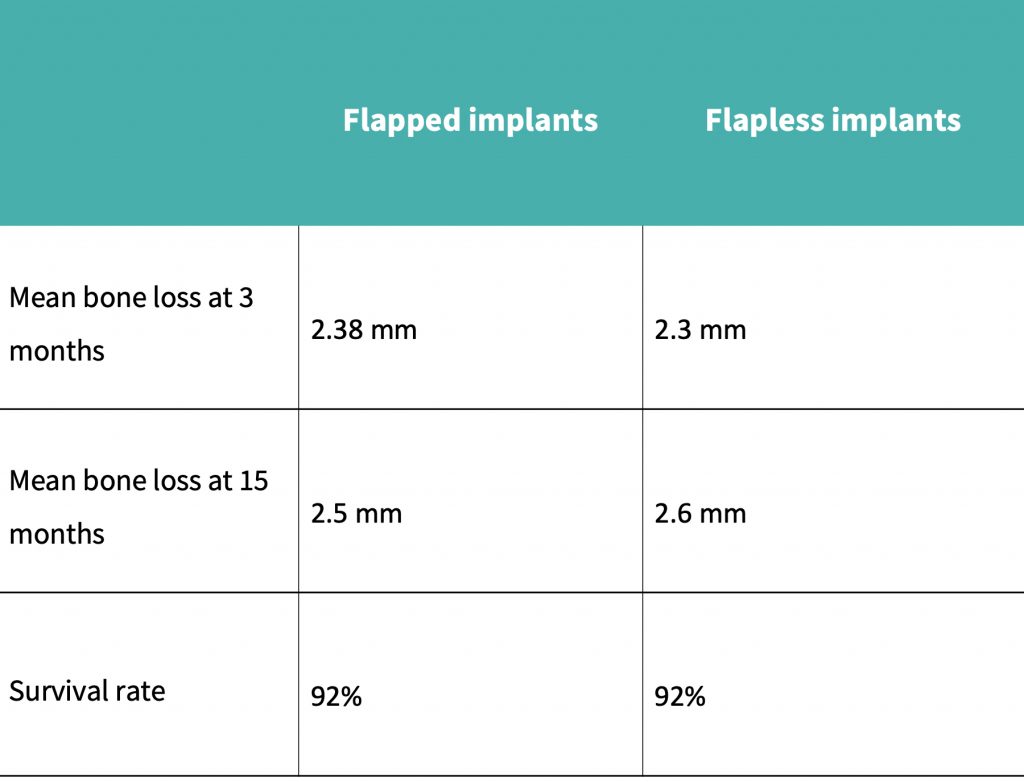
The study by Bashutski et al., (2013) showed that 1 implant had failed in each of the groups, and patients who had received flapped implant placement had an initial decrease in papillary index. In the flapped group, bone loss was more pronounced at 15 months after surgery, and keratinized mucosa levels had reduced more significantly in the flapped group than in the flapless group.
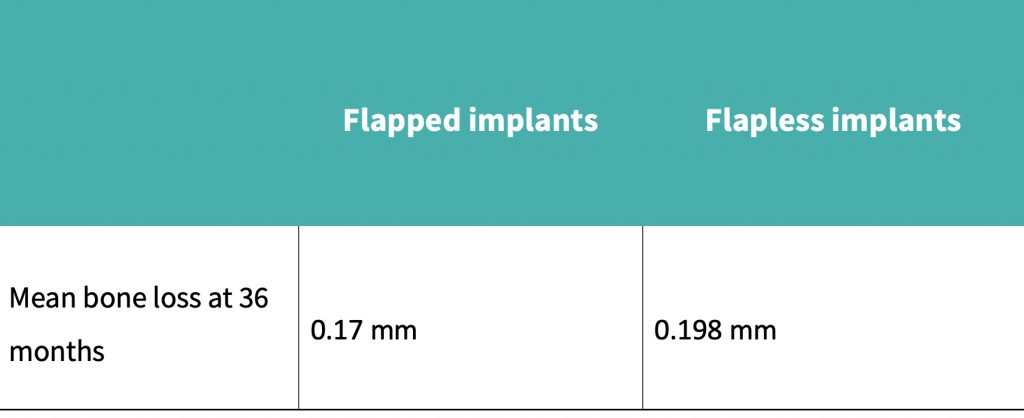
Pisoni et al. (2016) demonstrated that mean bone loss at 36 months with the flapped cohort was 0.17 mm, compared to 0.198 mm for the flapless group.
Conclusion
Publications exist that either claim flapless implant placement has positive impacts on biological tissue levels, or that it does not have major preservation potential. However, in my opinion, whatever the conclusion being touted by the research, the astute reader will pay careful attention to how the study is performed and the methodology followed, as many flaws have been detected throughout the literature review.
Within the limitations of this article and in my experience, we can conclude that although there is no major improvement in post-operative biological tissue levels when implants are placed flaplessly, rather than with flapped technique protocols, flapless implant placement may be more attractive, especially from a patient’s point of view. I would suggest that any elective surgery should minimize post-operative problems, and if flapless implant placement can harbor benefits, then it should be encouraged for the right cases, in my humble opinion.
However, I would argue that dentistry’s current level of evidence has failed to detect whether implants placed flaplessly support biological tissue preservation or not. Therefore, further large-scale human studies are needed with some attention paid to the following factors during the methodology: similar implant surfaces and types being used, stents used for intervention and control groups, similar surgical protocols and loading protocols, patients with similar medical histories to be included, correct and valid randomization and blinding strategies, and more long term data. The studies would also benefit from the integration of 3D CBCT imaging and clinical parameters such as bone sounding to paint a more accurate picture of the true quantity of bone loss around the implants during follow-up appointments.
Acknowledgements
I would like to express my most sincere gratitude to my tutors Drs. Zaid Alabdi and David Madruga for their help and support along the journey of my academic training in implant dentistry. I would also like to express my thanks to Drs. Rajan Patel, Sumair Khan and Kash Hafeez for supporting me in my clinical training.