Introduction
Dental implants for the replacement of missing teeth are rapidly increasing in popularity and demand by the general population. They represent an attractive treatment option for patients, both in anterior and posterior regions of the mouth. Replacing a missing tooth is vital for several reasons, which are commonly esthetically or functionally driven.
The second most common reason for tooth loss is periodontitis, with dental caries being number one. According to the Global Burden of Disease 2010, severe periodontitis was the sixth most prevalent condition in the world, accounting for approximately 11.2% of the world’s population, which is equivalent to 743 million people (Kassebaum et al. 2014). The prevalence of milder forms of periodontitis was estimated to be as high as 50% globally (Billings et al. 2018). Thus, it is very common for general dental practitioners (GDPs) to encounter patients requesting dental implant(s) with the concomitant presence of stable or unstable periodontitis. Therefore, careful consideration must be given to such a cohort of patients, in particular those with unstable periodontitis due to the increased risk of peri-implantitis demonstrated in longitudinal and cross-sectional studies (Ferreira et al. 2018; Roccuzzo et al. 2010; Schwarz et al. 2018). Coupled with the difficulty and unpredictability of treating peri-implantitis, this overview article highlights the paramount importance of diagnosis and treatment of periodontitis prior to implant placement.
Periodontitis
Periodontal diseases, an encompassing term, includes both gingivitis and periodontitis. Gingivitis is a reversible inflammatory condition of the gingival tissues associated with the accumulation of plaque (Loe et al. 1965). Periodontitis is a chronic multifactorial inflammatory disease associated with a dysbiotic plaque biofilm. This results in the progressive destruction of the periodontium characterized by the presence of periodontal pockets, clinical attachment loss and radiographic bone loss, and can ultimately result in tooth loss (Papapanou et al. 2018). Much attention is focused on the role of a dysbiotic plaque biofilm as an entity in periodontitis, along with a susceptible host associated with systemic risk factors such as uncontrolled diabetes mellitus and smoking (Lamont & Hajishengallis, 2015; Papapanou et al. 2018).
A periodontitis case may be clinically defined as (Tonetti et al. 2018):
- Interdental clinical attachment loss detectable at two or more non-adjacent teeth, or
- Buccal or oral clinical attachment loss ≥3 mm with pocketing ≥3 mmdetectable at two or more teeth, but the observed clinical attachment loss cannot be ascribed to non-periodontitis-related causes.
Diagnosis and classification of periodontitis are based upon a staging and grading. Staging is based on the severity and complexity of the disease ranging from stage I to IV. This is supplemented with a grade classification, which depicts the risk of disease progression and anticipated treatment response ranging from A to C (Papapanou et al. 2018; Tonetti et al. 2018). The extent of the disease is described by the number of teeth involved, categorized as either localized, generalized or molar-incisor pattern. The reader is referred to the 2017 World Workshop papers for more details with regards to the classification of periodontal and peri-implant diseases and conditions (Caton et al. 2018).
Periodontal therapy
Periodontitis, although a preventable and manageable disease, can result in detrimental effects. Tooth loss is the ultimate sequelae of the disease and, with reduced masticatory ability as a result, this subsequently leads to reduced nutrition, function and quality of life (Chapple 2014). Periodontal treatment aims to disrupt the microbial biofilm and re-establish a symbiotic relationship with the host to attain periodontal stability/health with tooth preservation, improved quality of life and an environment conducive to self-performed oral care. The goal of therapy is ‘pocket closure’ (no pockets >4 mm) and the absence of or a reduction in bleeding (Suvan et al. 2019; Tomasi et al. 2007). Table 1 defines stable and unstable periodontitis and gingivitis in periodontitis patients based on clinical parameters (Chapple et al. 2018).
| Stable periodontitis | Gingivitis in a periodontitis patient | Unstable periodontitis |
| PPD ≤ 4 mm | PPD ≤3 mm | PPD ≥5 mm or |
| No BOP at 4 mm sites | BOP ≥10% | PPD ≥4 mm with BOP |
| BOP <10% |
As per ‘The European Federation of Periodontology S3 Clinical Practice Guideline’, step 1 of therapy would typically include behavior change and motivation, oral hygiene instruction (OHI), control of risk factors and supra-gingival dental biofilm control (Sanz et al. 2020). This is followed by step 2 which involves sub-gingival instrumentation, and if periodontal pockets persist then step 3 would include re-instrumentation or possible surgery. It is advised for periodontal surgery to be undertaken by specialists or those with a special interest in periodontology. Finally, successfully treated periodontal patients should be enrolled into a tailored and appropriate supportive periodontal care (SPC) regime which encompasses steps 1 and 2 above. An overview of periodontal treatment sequences is illustrated in Table 2.
| Step 1 | Step 2 | Step 3 | SPC |
| Oral hygiene instruction | Subgingival biofilm and calculus control | Repeated subgingival instrumentation with or without adjunctive therapies | Maintain periodontal stability |
| Motivation and coaching | Subgingival instrumentation | Periodontal surgery o Access flap o Resective o Regenerative | Regular recall visits |
| Risk factor control | With or without adjunctive therapies | Prevention and treatment as necessary (Step 1 and 2) | |
| Supragingival plaque control |
*Step 1 should be reinforced at all stages of treatment
Prevalence of peri-implantitis in periodontitis patients
Patients with a history of periodontitis, and more specifically unstable periodontitis, are more likely to experience peri-implantitis. Of note, varying odds ratios were reported in multiple studies; a meta-analysis based on observational studies reported patients with a history of periodontitis were 2.3 times more likely to have peri-implantitis (Ferreira et al., 2018; Roccuzzo et al. 2010). Furthermore, patients with an initial diagnosis of severe periodontitis (equivalent to stage III or IV), when compared to healthy individuals, demonstrated a profoundly higher frequency of implant sites with bone loss (27% and 2%, respectively) and deep pocketing (15% and 5%, respectively) at 10-year follow up (Roccuzzo et al. 2010). In brief, there is consistent evidence showing patients with a history of periodontitis having a significantly higher risk for developing peri-implantitis compared to healthy individuals (Schwarz et al. 2018).
Risk indicators for peri-implant diseases
1. Residual periodontal pockets of ≥5 mm
The goal of treatment for periodontitis is the closure of periodontal pockets to below 4mm without bleeding on probing (Caton et al. 2018; Lang & Bartold 2018). Patients with unstable periodontitis, classified by residual pocketing of ≥ 5 mm, demonstrated a significantly higher risk for peri-implantitis compared to stable periodontitis patients (Pjetursson et al. 2012). A retrospective study found that the prevalence of peri-implantitis to be 30% and 12%, for unstable and stable periodontitis patients, respectively (Cho-Yan Lee et al. 2012). Increased bone loss and deeper probing depths around implants were detected more frequently in unstable periodontitis patients. An additional study showed the incidence of peri-implant mucositis converting to peri-implantitis was 16 times more likely in patients with a higher prevalence of sites greater than 4mm (Costa et al. 2012). Consequently, reducing the number of periodontal pockets and maintaining a stable gum health will reduce the risk of peri-implantitis.
2. Oral hygiene
Poor oral hygiene and irregular attendance during maintenance (poor compliance) is attributed to disease progression. One of the main reasons for stabilizing periodontitis prior to implant placement is to ensure patients acquire the skills and knowledge to successfully maintain an optimal level of oral hygiene. It is well established that optimal oral hygiene is of utmost importance in maintaining periodontal health (Axelsson et al. 2004; Magnusson et al. 1984). In general, a full-mouth plaque score of less than 20% is essential for stable periodontal health over the long-term (Axelsson et al. 2004). This is also true for peri-implant health as a significant dose-dependent relationship between plaque accumulation and peri-implant mucositis has been established (Ferreira et al. 2006). By improving the patient’s oral hygiene, specifically around implants, one can reduce the risk of peri-implant mucositis. This would be in tandem with correcting any necessary local factors which may impede the patient’s ability to clean around the implants. As a direct effect, this would translate to a lower incidence of peri-implantitis, as persistent inflammation around an implant demonstrated an approximately 18 times higher incidence of conversion to peri-implantitis (Costa et al. 2012; Lang & Berglundh 2011).
3. Compliance with maintenance visits
One of the critical elements in the success of periodontal therapy is adherence to a maintenance regime. Even with successful periodontal treatment, periodontitis patients demonstrated significantly higher disease recurrence with poor compliance (Lindhe & Nyman 1984). Patients who adhered to a tailored recall program were more likely to maintain periodontal health, good oral hygiene and minimize tooth loss in the long term (Axelsson et al. 2004; Hirschfeld & Wasserman 1978). This is similar in patients with implants where the prevalence of peri-implantitis was reduced from 44% to 18% in those who were compliant with supportive care (Costa et al. 2012). Furthermore, patients with lack of compliance to supportive implant therapy were six times more likely to develop peri-implantitis from a condition of peri-implant mucositis (Costa et al. 2012). With increased risk of peri-implantitis in those who fail to comply with maintenance visits, this can result in substantially higher implant loss and need for further treatment (Pjetursson et al. 2012; Roccuzzo et al. 2010). Of note, implants placed in patients who had received active periodontal therapy followed by stringent maintenance demonstrated success rates comparable to healthy individuals (Tan et al. 2017). Hence, good adherence to maintenance visits is imperative for stable periodontal and peri-implant health.
4. Systemic risk factors
The initial phase or “Step 1” of periodontal therapy involves controlling recognized risk factors, such as smoking and diabetes mellitus. Currently, the evidence for the association of peri-implantitis with smoking and diabetes mellitus remains inconclusive, although this does not preclude them as risk factors (Schwarz et al. 2018). Existing evidence shows patients who smoke or with uncontrolled diabetes mellitus tend to have a sub-optimal healing and treatment response compared to healthy individuals (Preshaw et al. 2005; Rosa et al. 2014; Taylor 2001). Controlling the patient’s risk factors prior to implant treatment is highly recommended as it reduces the risk profile.
Furthermore, informing and educating the patient is a crucial part of helping to control the risk factors. This is as important in both periodontitis and non-periodontitis susceptible individuals receiving implants. Patients should be made aware of the risk of implant failure which can lead to its loss, just like natural teeth, and this risk tends to be higher in unstable periodontitis patients. This facilitates gaining the appropriate informed consent and also reinforces the importance of the patient taking ownership of their oral health.
Implant treatment in periodontal patients
Ideally, a state of periodontal stability and control of risk factors is to be achieved prior to commencing implant treatment (Chapple et al. 2018). Nevertheless, this is not always achievable, and a holistic approach must be adopted on a case-by-case basis. This would include appreciation of multiple factors such as pocket depth, the site of the periodontally affected tooth/teeth, the presence of systemic and local risk factors and the level of oral hygiene. Although there is no set threshold for suitability for implant therapy, one must strive for periodontal stability where appropriate. Periodontal stability and SPC ought to be carried out and maintained for a reasonable period of time where the patient demonstrates compliance, motivation and the ability to maintain an optimum level of oral hygiene. However, there is no hard and fast rule for the length of this ‘maintenance period’. This is because periodontitis is a chronic condition with the interplay of various local and systemic risk and prognostic factors in the disease process (Fu & Wang 2020).
For this reason, a tailored personalized SPC regime should be unique to each individual patient based on their treatment needs and risk profile. One tool which may assist the clinician in evaluating this risk is the “Periodontal Risk Assessment (PRA)” (N. P. Lang & Tonetti 2003). This risk assessment tool considers numerous parameters for the risk of disease progression in patients in SPC. The risk level is defined as low, moderate or high. Suggested recall time intervals based upon the risk category can be formulated (high risk tending to be every 3 months) (Trombelli et al. 2020). Those patients at high risk may need to be followed more frequently and for longer during this ‘maintenance period’ compared to patients with a low risk. The reader must be told that this tool is not a guide for the timing of implant placement, but for assessing the level of risk for a patient. Hence, it is a useful guide in the management of periodontal patients.
Conclusion
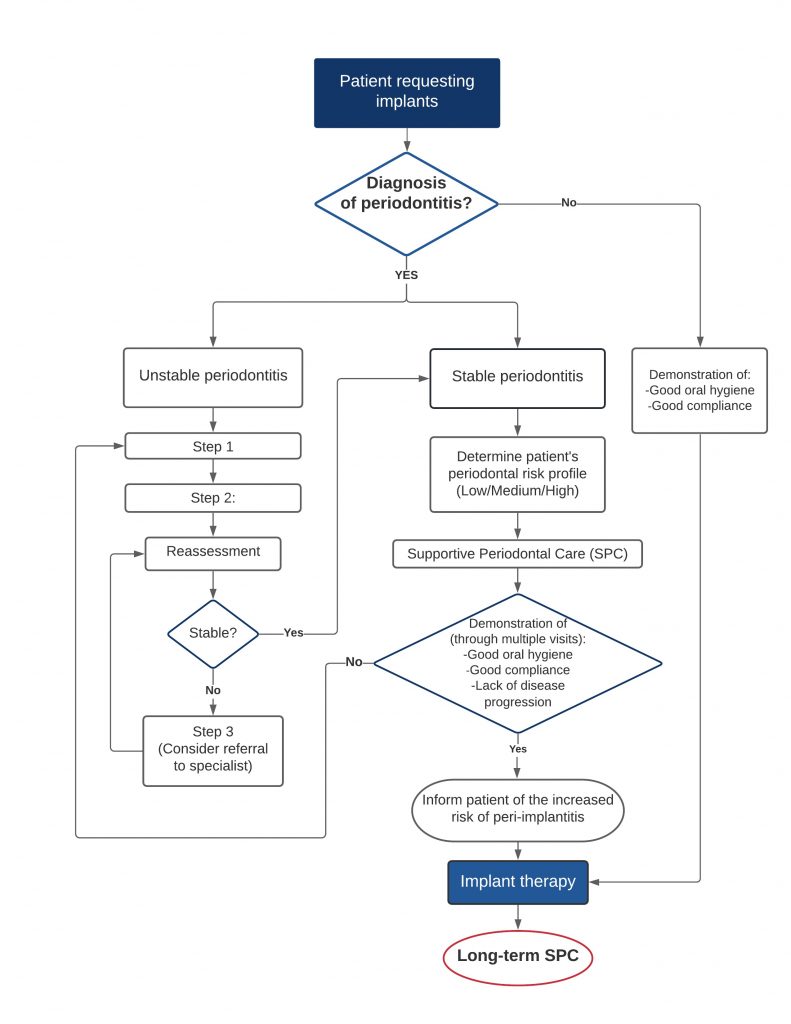
Risk assessment of each patient for their suitability for implants and the predictability for success of treatment is extremely important. Furthermore, it is vital to establish whether the patient is a periodontitis patient and whether further treatment is needed to stabilize disease progression and attain periodontal health. This is because unstable periodontitis results in increased risk of peri-implantitis and therefore possible implant failure and ultimately loss of the implant. Not only is the management of periodontitis imperative to attain health but also long-term maintenance is key to sustained stability. This means a tailored SPC regime in which the patient demonstrates compliance is critical for the long-term success of implants. The above prior management would support the placement of implants with the reduced risk of peri-implant diseases. Please refer to Fig. 3 depicting a summary flowchart for the management approach for patients requesting dental implants (authors’ opinion).
Take home messages:
- Ensure a diagnosis of periodontitis has been established and appropriate treatment provided.
- A successfully treated periodontitis patient remains a periodontitis patient for life due to the risk of disease recurrence.
- Inform patients with a history of periodontitis of the increased risk of peri-implant diseases (particularly those non-compliant with SPC).
- A period of periodontal stability prior to implant placement is recommended.
- SPC is key to periodontal and implant health.