What is this blog about?
Oral neutrophil levels may potentially reflect the severity of peri-implant disease (PID) and treatment response. A single, rapid oral rinse assay is an effective means of collecting and quantifying oral neutrophil levels and may serve as an excellent tool for assessing the role of neutrophils and associated inflammatory markers in peri-implant diseases.
What is the role of neutrophils in PID?
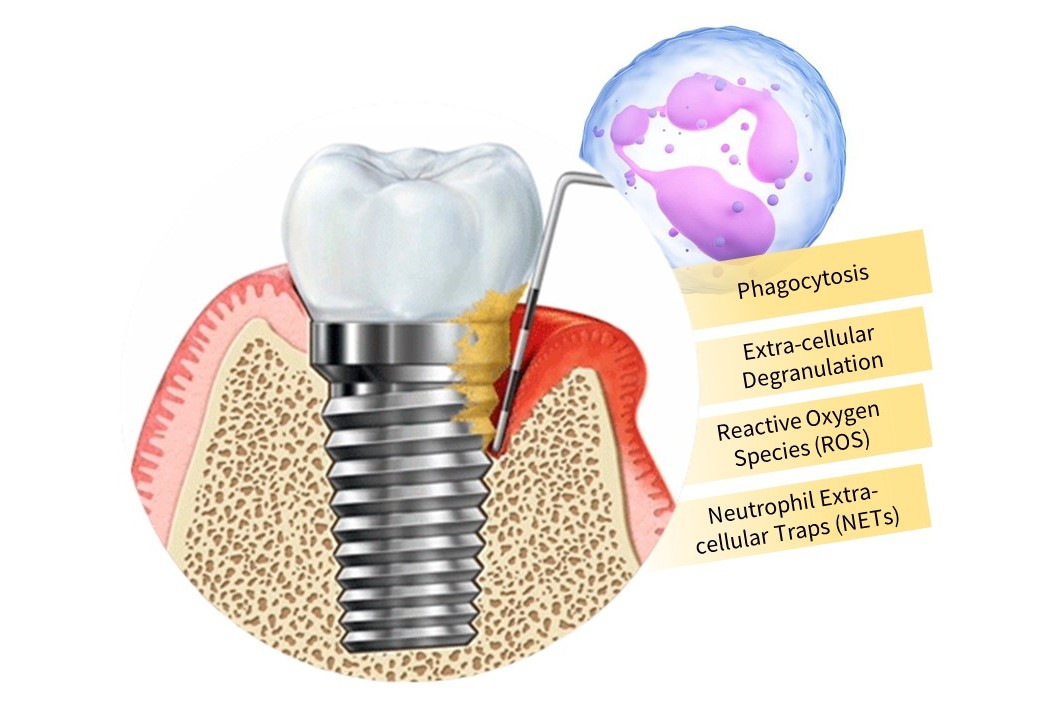
Periodontitis and peri-implantitis share clinical, radiological, histological, and histomorphometric similarities with respect to etiology and clinical features but evidence supports the differences in pathogenesis. Although the early host response to the bacterial infiltration is identical, it has been established that the apical extension and the size of the inflammatory infiltrate are more pronounced in the peri-implant mucosa than in the gingiva. This indicates a stronger host response to the bacterial challenge in the soft tissues adjacent to implants than around natural dentition. Inflammatory homeostasis of periodontal tissues is maintained by oral polymorphonuclear leukocytes (oPMNs) through several antimicrobial mechanisms, including phagocytosis, extracellular degranulation, the release of reactive oxygen species (ROS), and formation of neutrophil extracellular traps (NETs). In the context of biofilm dysbiosis, PMNs become hyperactive and undergo amplified recruitment which leads to gingival inflammation and progressive loss of periodontal attachment.
In the presence of chronically increased pathogen levels, oPMN counts are elevated to combat the invading microbes. However, if sustained, they help to propagate periodontitis or at least the tissue destruction observed in patients affected by this condition. Since, oPMNs play a role in the pathogenic mechanisms of PID similar to those for periodontal diseases, it stands to reason that oPMN counts and PMN markers of activation may be of diagnostic utility. In addition to serving as possible diagnostic and treatment-monitoring markers, the oral neutrophil activation state could serve as a tool to study the role of neutrophils in maintaining health at the peri-implant tissue attachment and to better understand their role in inflammatory PIDs.
How do we clinically correlate neutrophils and their markers to the severity of PIDs?
Glogauer and co-workers (2009) have developed a method for diagnosing and monitoring periodontal diseases. Oral PMNs of participants are analyzed using a non-invasive rinse diagnosis method. Samples are collected by means of 10 sequential oral rinses over a 30-minute period using 0.9% NaCl with subsequent expectoration into a sterile falcon tube; stored at 4ºC and tested within 2 hours of procurement. This method permits the identification of functional and surface marker changes on PMNs, thereby providing information to assess the periodontal inflammatory status and disease activity of an individual subject. The novelty of this technique is as a screening tool that can be utilized practically in combination with clinical examination to assess underlying disease activity and disease progression in periodontal tissues. The major advantages of this test include its ease of use, cost-effectiveness and non-invasiveness. We propose that this tool may also aid in assessing disease progression and treatment response in patients with peri-implantitis.
How did we conduct the pilot study to correlate neutrophils and their markers to the severity of PIDs?
Using this protocol, the following parameters can be assessed from an oral rinse sample:
- Oral inflammatory load (OIL)PMN surface markers [degranulation/activation markers (CD10, CD63, CD64, and CD66a), immunoregulation markers (CD16 and CD170), adhesion markers (CD11b, CD18, and CD177), and complement regulators (CD55)]
- Reactive oxygen species (ROS) assay
- Neutrophil extracellular trap (NET) Assay
Oral inflammatory load (OIL)
Epithelial cells were removed from these samples by filtration to isolate highly pure PMN populations. Rinse samples were centrifuged at 2500 RPM for 5 minutes and viable cells were collected. One ml of the suspension was removed and 4 µl of acridine orange was added to the cells. This allowed neutrophils to be distinguished from other cells using fluorescence microscopy. A 10 µl aliquot of this suspension was then assessed on a hemocytometer, and the PMNs were counted visually using fluorescence microscopy.
Multicolor Flow Cytometry
To examine PMN surface-marker expression, PMNs were resuspended in FACS buffer and labeled with two separate panels of antibodies. The markers are classified into 4 categories based on function: degranulation/activation markers (CD10, CD63, CD64, and CD66a), immunoregulation markers (CD16 and CD170), adhesion markers (CD11b, CD18, and CD177), and complement regulators (CD55). For each CD marker, appropriate fluorescently tagged isotype control antibodies were used to determine auto-fluorescent signals, which were subtracted from mean fluorescence intensities (MFIs).
Reactive oxygen species (ROS) assay and Neutrophil extracellular trap (NET) Assay
For ROS, unfixed PMNs were prepared and incubated for 20 minutes at 37ºC in Hanks solution containing dihydrorhodamine at a final concentration of 2 μM and stimulated for an additional 15 minutes with phorbol 12-myristate 13-acetate (PMA) at a final concentration of 200 nM or left unstimulated. For NET, a preparation of fixed, purified oral PMNs was performed. These PMNs were labeled sequentially with primary anti-Histone H3 and secondary goat anti-rabbit-AF488 anti-bodies, then labeled with MPO-PE and CD18-BV421 and analyzed. Flow cytometric analysis was performed to assess ROS and NET.
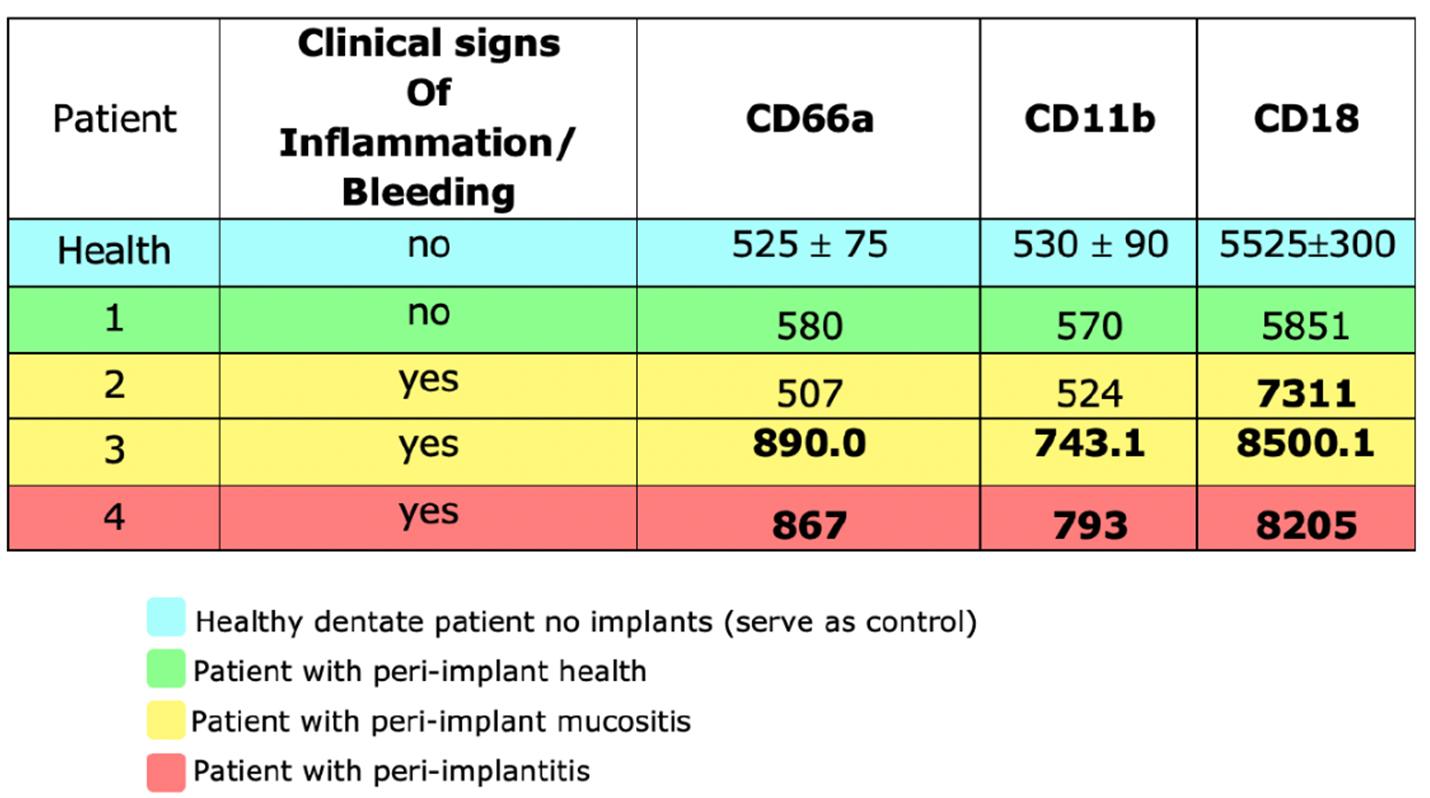
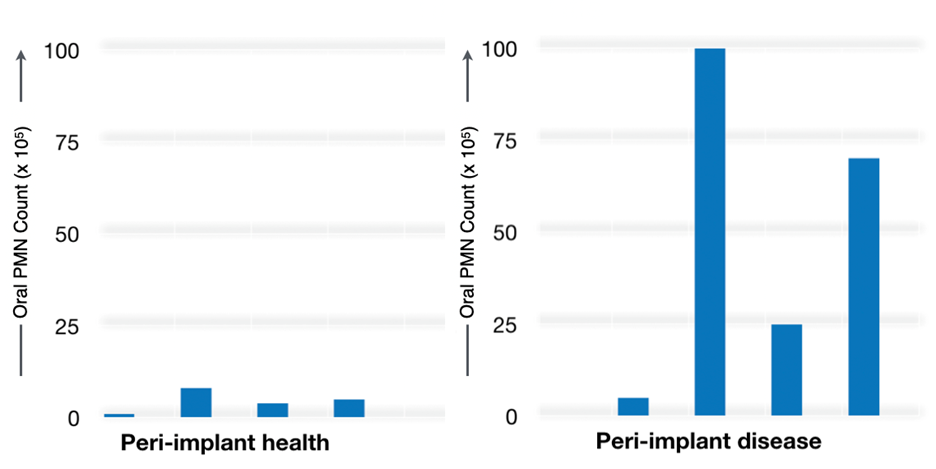
In this pilot study, we found that patients with clinical and radiographic PIDs had an elevated OIL, as demonstrated by a 10-fold increase in oPMN counts. The mean expression of CD markers in a healthy dentate patient was assessed independently and used as a control. A higher surface expression of CD markers CD66a, CD11 and CD18 was observed in PIDs as compared to healthy peri-implant tissue, suggesting a marked inflammatory response in PID.
Conclusion
The results of our pilot study suggest that the activation state of oral PMNs correlates positively with the severity of peri-implantitis as compared with those in healthy peri-implant tissues. Moreover, a higher surface expression of CD markers on salivary neutrophils in peri-implantitis indicates that quantification these markers can provide a valuable screening and monitoring tool for peri-implantitis.