Introduction
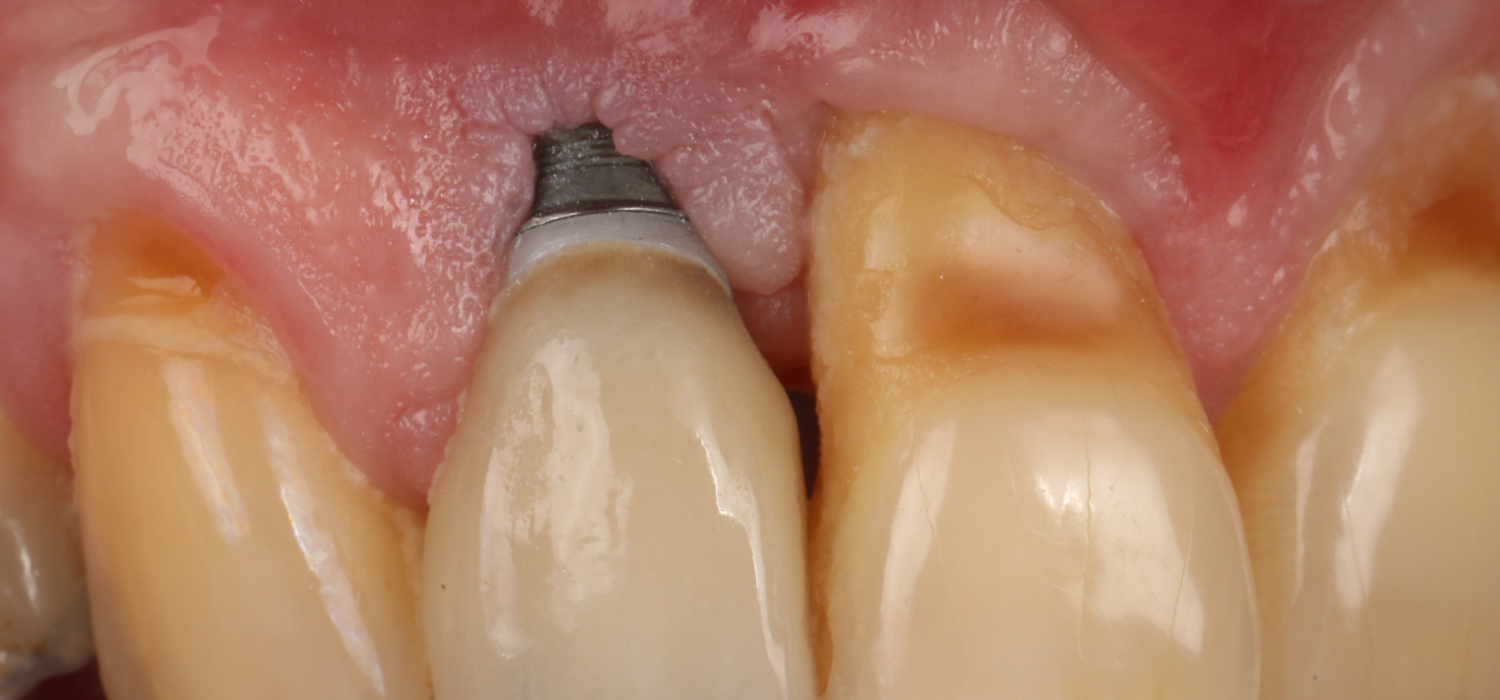
Implant therapy is a predictable treatment option for replacing missing and/or terminal dentition and it has been proven to provide satisfying outcomes in the long term. Nonetheless, there are some complications related to implant therapy that can occur over time. Among them, esthetic complications are usually the most noticeable for patients (Zucchelli et al. 2020). Indeed, these conditions can be characterized by a very long implant-supported crown and/or by an apical shift of the soft tissues, revealing the metal components of the dental implant (Figure 1). These esthetic complications can impair patients’ perception of implant therapy and possibly leave a negative impact on their quality of life (Anderson, Inglehart, El-Kholy, Eber, & Wang, 2014; Zucchelli et al. 2019).
There are several factors that can contribute to a higher risk of implant esthetic complications, including inadequate keratinized mucosa width and soft tissue thickness (Lin, Chan, & Wang, 2013; Sanz-Martin et al. 2020; Tavelli et al. 2022), buccally positioned implants (Sanz-Martin et al. 2020), bony dehiscence or fenestrations (Hammerle & Tarnow, 2018; Tavelli et al. 2022) and multiple adjacent dental implants (Romandini et al. 2020; Sanz-Martin et al. 2020), among others. Given all of these risk factors, it is not surprising that the incidence of implant esthetic complications has been estimated to be 54.2% at the individual level and 56.8% at the implant level (Tavelli et al. 2022).
Treatment goals and approaches for the management of implant esthetic complications
When it comes to the management of implant esthetic complications, it has been advocated that the goals of the intervention should be: i) correction of the soft tissue defect, meaning repositioning the soft tissue margin coronally at the level of the homologous natural tooth, ii) recreation of an adequate dimension of peri-implant soft tissues (keratinized mucosa width and mucosal thickness), and iii) patient satisfaction and confidence with the esthetic outcomes.
Several approaches have been described for the management of esthetic complications, including the coronally advanced flap in combination with a connective tissue graft, the tunnel technique, the VISTA approach, free gingival grafts, guided bone regeneration procedures, the resubmergence technique, the surgical-prosthetic approach, and the connective tissue graft-platform approach (Anderson et al. 2014; Burkhardt, Joss, & Lang, 2008; Mazzotti et al. 2018; Stefanini, Marzadori, Tavelli, Bellone, & Zucchelli, 2020; Tavelli, Majzoub, et al. 2023; Tavelli, Zucchelli, et al. 2023; Zucchelli et al. 2013).
The indication for these techniques largely depends on the characteristics of the implant esthetic complications, which include the height of the implant-supported crown, the three-dimensional position of the implant, and the dimension of the papillae, among other factors. When appraising the existing literature on this topic, it should be acknowledged that most of the studies are case reports or case series, and therefore performing systematic reviews and meta-analyses on the management of implant esthetic complications may be inconclusive at this point.
Coronally advanced flap vs tunnel technique with a connective tissue graft for the treatment of isolated esthetic complications
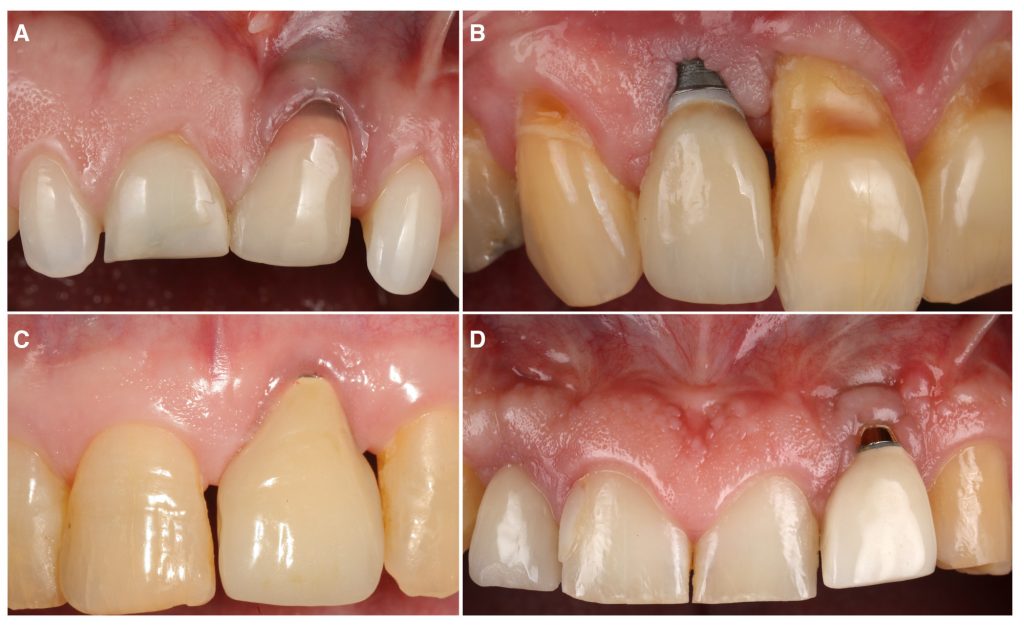
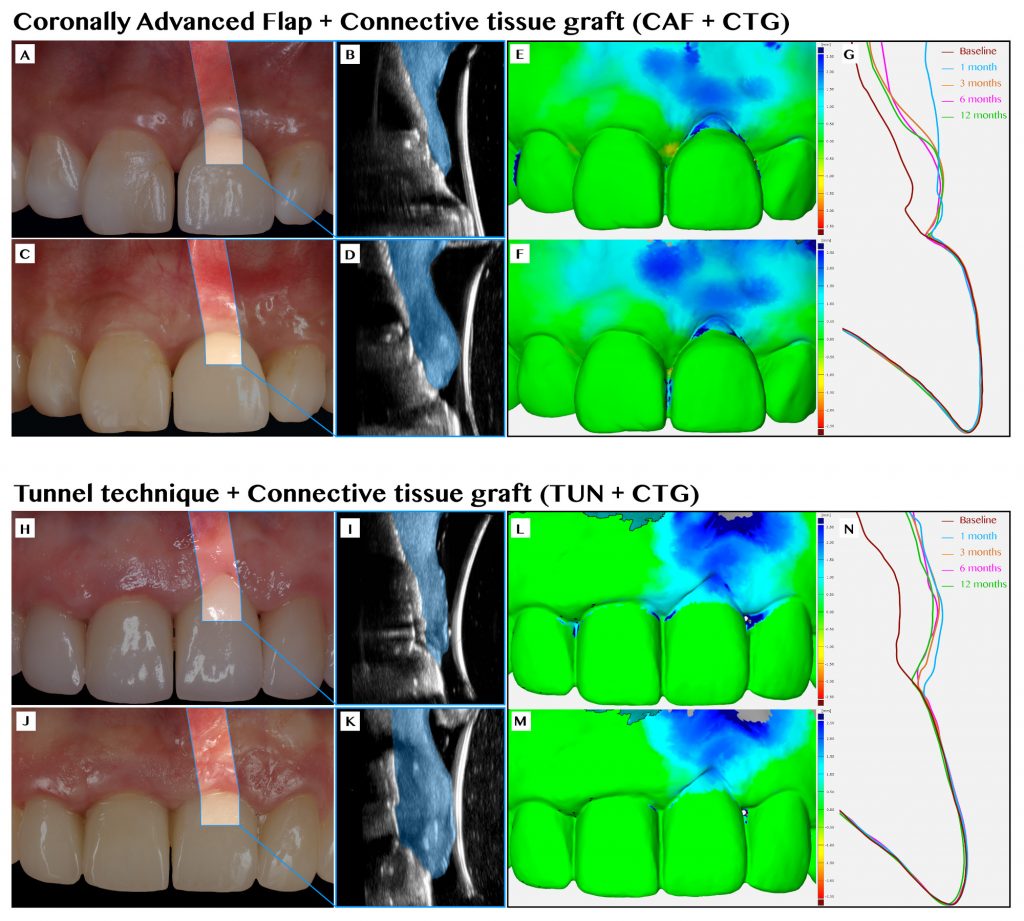
Our group recently published the first randomized controlled clinical trial on this topic, with the aim of comparing the efficacy of two commonly performed techniques, coronally advanced flap (CAF) and tunnel technique (TUN), both in combination with a connective tissue graft (CTG), for the treatment of single peri-implant soft tissue dehiscences (Tavelli, Majzoub, et al. 2023). Twenty-eight patients were randomized and treated with either envelope CAF + CTG or TUN + CTG, and followed for 12 months. The mean coverage of the defect was 90.23 ± 19.85% and 59.76 ± 34.94% for the sites treated with CAF and TUN, respectively. This difference between the two groups was statistically significant (p=0.03). Moreover, more implants treated with CAF showed complete resolution of the esthetic complications, compared to the sites allocated to TUN (complete dehiscence coverage of 71.4% for CAF and 28.6 for TUN, p=0.07). In addition to these outcomes, we assessed the changes in the volume of the soft tissue using conventional transmucosal piercing, the superimposition of digital impression models, and ultrasonography. After 1 year, we observed a superior mucosal thickness gain for the sites that were treated with CAF compared to TUN (on average, 1.48 mm vs 1.02 mm, p=0.02). The superimposition of the intraoral scans showed that implants allocated to CAF had a higher volumetric gain than implants treated with TUN (on average, 79.73 mm3 vs 39.41 mm3, p<0.01). Another interesting finding was the superior gain in keratinized mucosa width that was observed in the CAF group (on average 2.57 mm, compared to a mean gain of 1.57 mm of the TUN group, p=0.01).
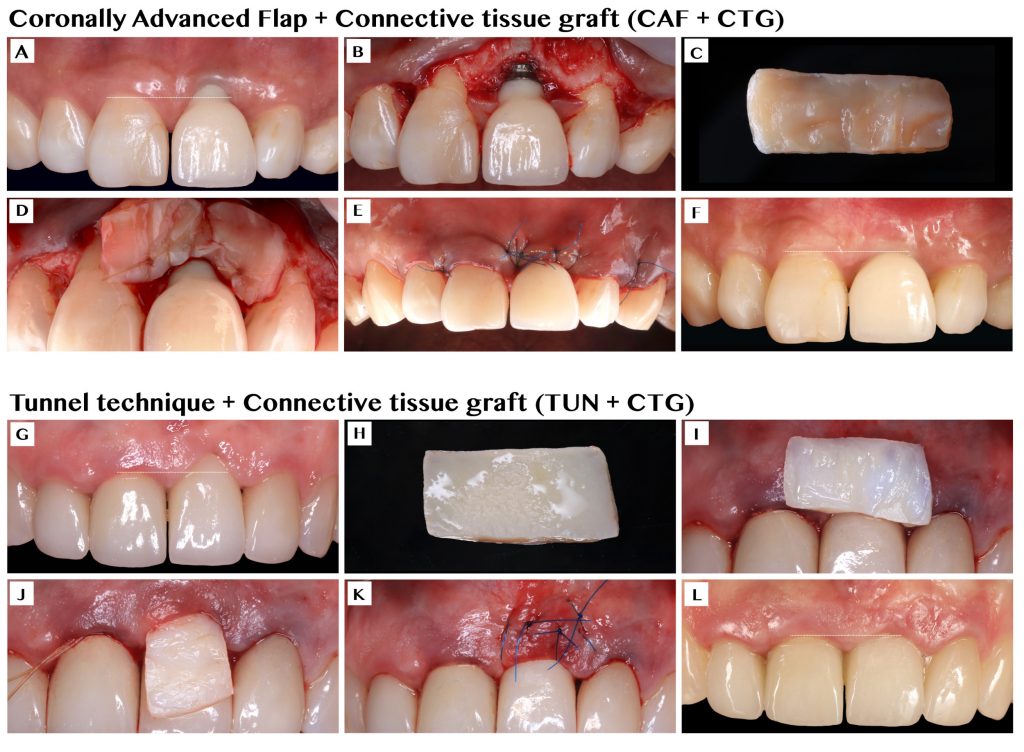
Although both groups received the same graft material (CTG), the differences between the two groups are open to speculation. Our group suggests that CAF allows for a higher stabilization of the CTG compared to TUN, which may explain the greater increase in soft tissue thickness/volume and keratinized mucosa width (Zucchelli, Tavelli, Stefanini, Barootchi, & Wang, 2021).
It is also important to highlight that both groups demonstrated a significant improvement in patient-reported outcomes, such as esthetics, satisfaction, and reduction of the anxiety related to the appearance of the implant (Tavelli, Majzoub, et al. 2023).
Doppler ultrasonography and future research
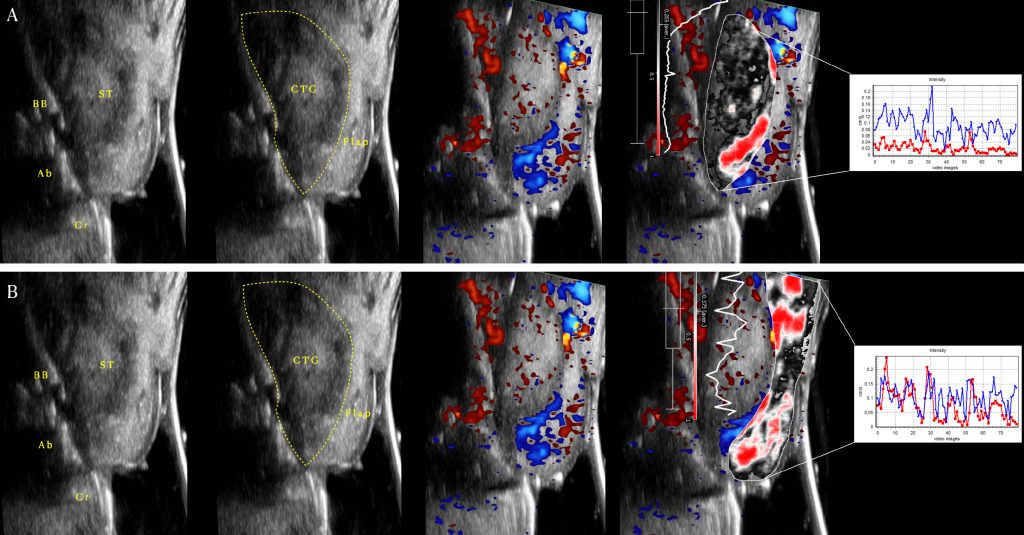
In the same patient population, we also applied doppler ultrasonography to assess – in a real time and non-invasive way – the changes in the blood flow of the soft tissue over time and the process of the revascularization of the CTG. We observed that at early time points (1 week and 1 month), most of the blood supply of the graft came from the overlying flap. In addition, we demonstrated that the higher the revascularization of the CTG at 1 week, the greater the final peri-implant soft tissue thickness at 1 year. This observation may further support the speculation that the stabilization of the CTG is crucial to allow quick revascularization and healing, with possibly less shrinkage of the graft (Tavelli, Kripfgans, et al. 2023).
Future clinical trials assessing different surgical approaches, and biomaterials, with a medium and long-term follow-up are required to further assess the efficacy of soft tissue augmentation for the treatment of implant esthetic complications. The application of technologies, such as ultrasonography and intraoral optical scanners, is encouraged to study the healing of soft tissue grafting at implant sites, and the treatment outcomes of these approaches for the correction of implant esthetic complications.
Conclusions
Implant esthetic complications are not rare. Soft tissue augmentation with a connective tissue graft is a valid approach for treating these conditions. In our recent clinical trial, we found superior clinical and volumetric outcomes for the coronally advanced flap over the tunnel technique. Novel non-invasive technologies can provide novel insights and findings related to soft tissue grafting at implant sites and, as such, are highly advocated to advance the field.